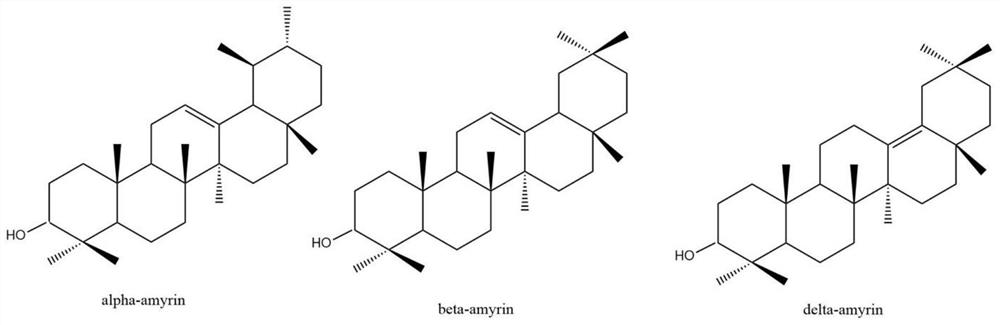
Protein for catalytic synthesis of amyrin, and preparation method and application for protein
A kind of technology of amyrin and protein, applied in the direction of biochemical equipment and method, botany equipment and method, application, etc., can solve the problem of not separating multifunctional OSC enzyme, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The embodiment of the present invention provides a method for preparing a protein used to catalyze the synthesis of myringin, which includes the following steps.
[0049] (1) Acquisition of ItOSC2 coding gene
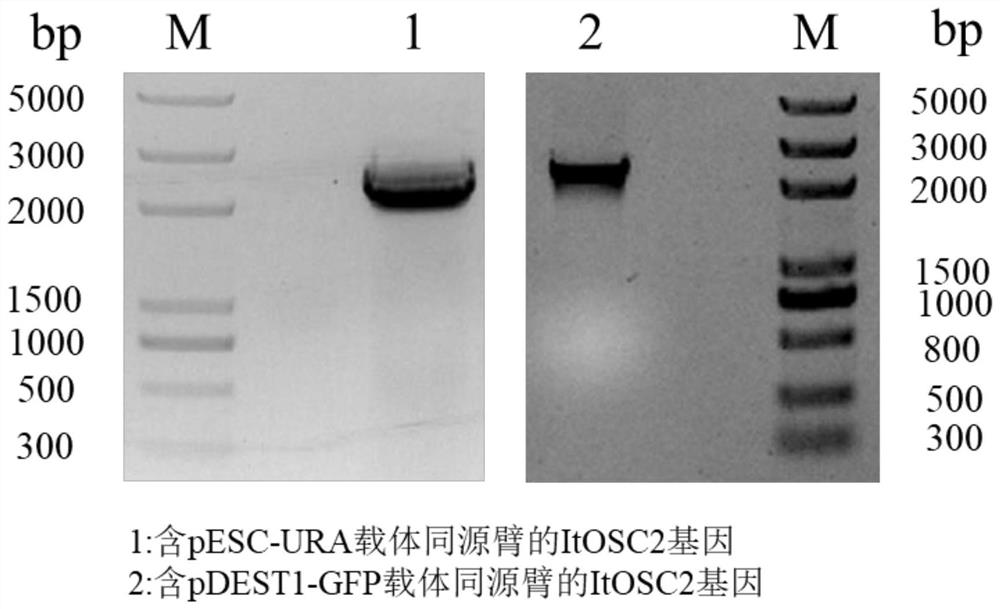
[0050] Iris (Iris tectorum Maxim) leaf total RNA was used as a template, and two primer pairs whose sequences were shown in Table 1 were used for RT-PCR amplification respectively. Specifically, the primers include a sequence of the candidate gene and a homology arm sequence on the pESC-URA or pDEST1-GFP vector, the lowercase letters indicate the homologous sequence of the vector, and the uppercase letters indicate the sequence of the gene. Specifically include the following steps.
[0051] Table 1 Primer Sequence
[0052]
[0053] In Table 1, F is an upstream primer, and R is a downstream primer.
[0054] 1. Extraction of total RNA
[0055] Apply the Rapid Universal Plant RNA Extraction Kit 3.0 (product number: 0416-50) of Beijing Huayueyang Biotechnology...
Embodiment 2
[0075] Obtaining and expressing ItOSC2 tobacco expressing plants.
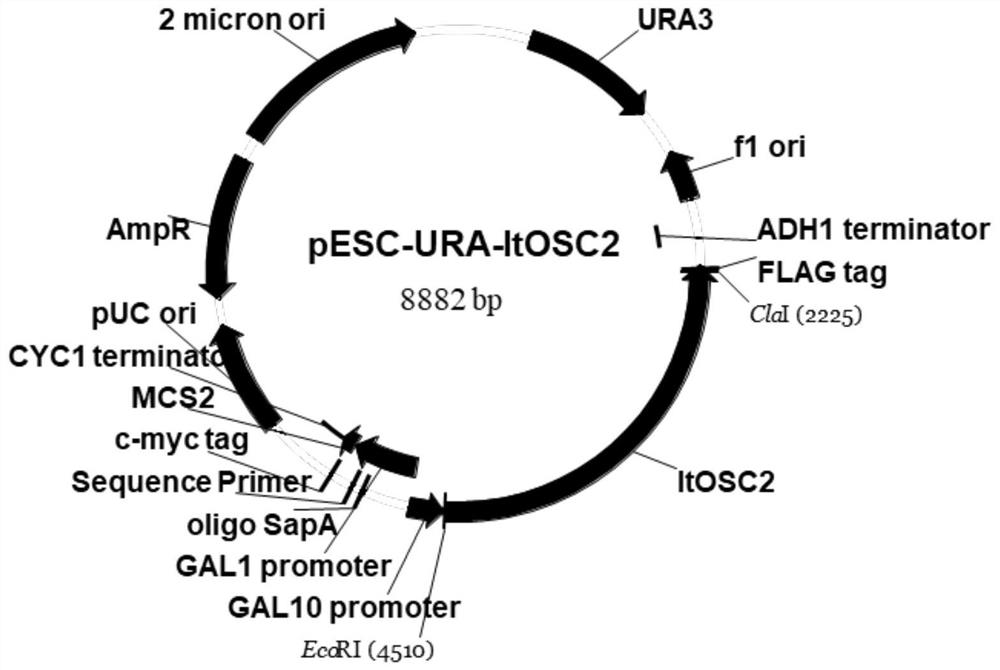
[0076] 1. Construction of tobacco expression vector pDEST1-GFP-ItOSC2
[0077] pDEST1-GFP was double digested with restriction enzymes FastDigest Mlu I and FastDigest Xho I (Thermo Fisher Scientific). The digestion system is: 3 μL 10× FastDigest Buffer, 3 μL FastDigestMlu I (FD0564), 3 μL FastDigest Xho I (FD0694), 2.4 μg pDEST1-GFP, plus ddH 2 Add O to 30 μL of the system, and flick to mix well; the reaction conditions are: 37°C, 1h. After the enzyme digestion was completed, it was detected by 1% agarose gel electrophoresis (100V, 30min), and the enzyme-digested vector was recovered by cutting the gel.
[0078] The recovered fragment of ItOSC2 (Example 1) was homologously ligated with the double-digested product. The ligation system is: 2 μL pDEST1-GFP double enzyme digestion product, 3 μL gel recovery product, 2 μL 5×CEⅡ Buffer, 1 μL Exnase Ⅱ, add ddH 2 Supplement O to 10 μL of the system, gently pipette...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com