Temperature-sensitive spiral polyisocyanide derivative constructed by dynamic acylhydrazone bond and preparation method thereof
A temperature-sensitive helix and acylhydrazone bond technology, applied in the field of bionic intelligent materials, can solve problems such as difficulties and limited applications, and achieve the effects of low cost, simple and easy method, and excellent temperature-sensitive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
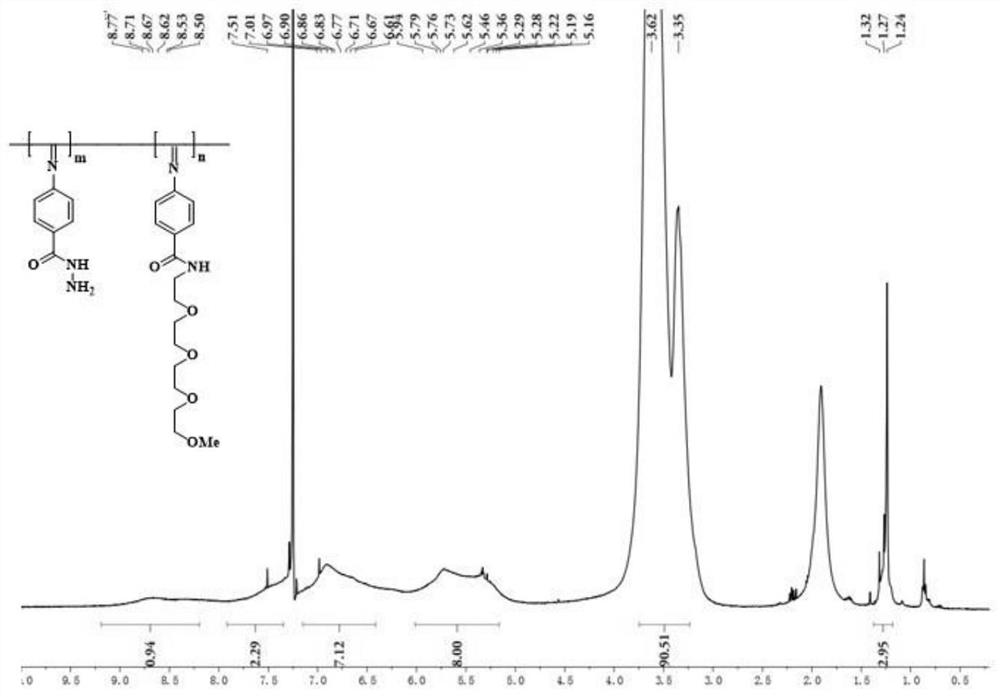
[0034] In this embodiment, a method for preparing a temperature-sensitive helical polyisocyanate derivative constructed by a dynamic acylhydrazone bond, the steps are as follows:
[0035] a. Dissolve the methyl-terminated oligomeric alkoxyether base and 4-formamidobenzoic acid in DCM at a molar ratio of 1.25:1 after amino modification, carry out ice-salt bath to the mixed solution, and inert Under gas protection, 1-hydroxybenzotriazole (HOBt) was added in the mixed solution, and after stirring for 30 minutes, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride ( EDC·HCl), its dosage is 1 time of the molar weight of the linear alkoxy ether unit; stir until the reaction is complete, after separation and purification, the obtained monomer and triethylamine are dissolved in DCM, and triphosgene is dissolved in In DCM, it was slowly added dropwise to the mixed liquid reaction system in an ice-salt bath, and after 30 minutes of reaction, NaHCO 3 solution, heated to 0°C, stir...
Embodiment 2
[0049] This embodiment is basically the same as Embodiment 1, especially in that:
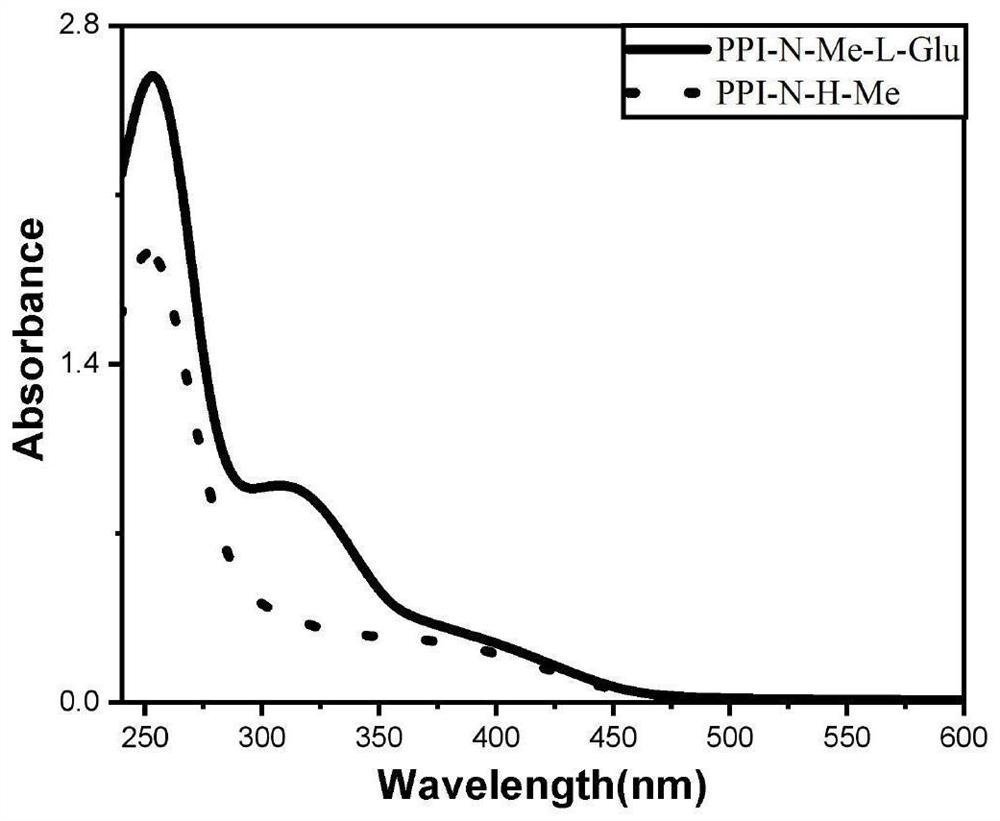
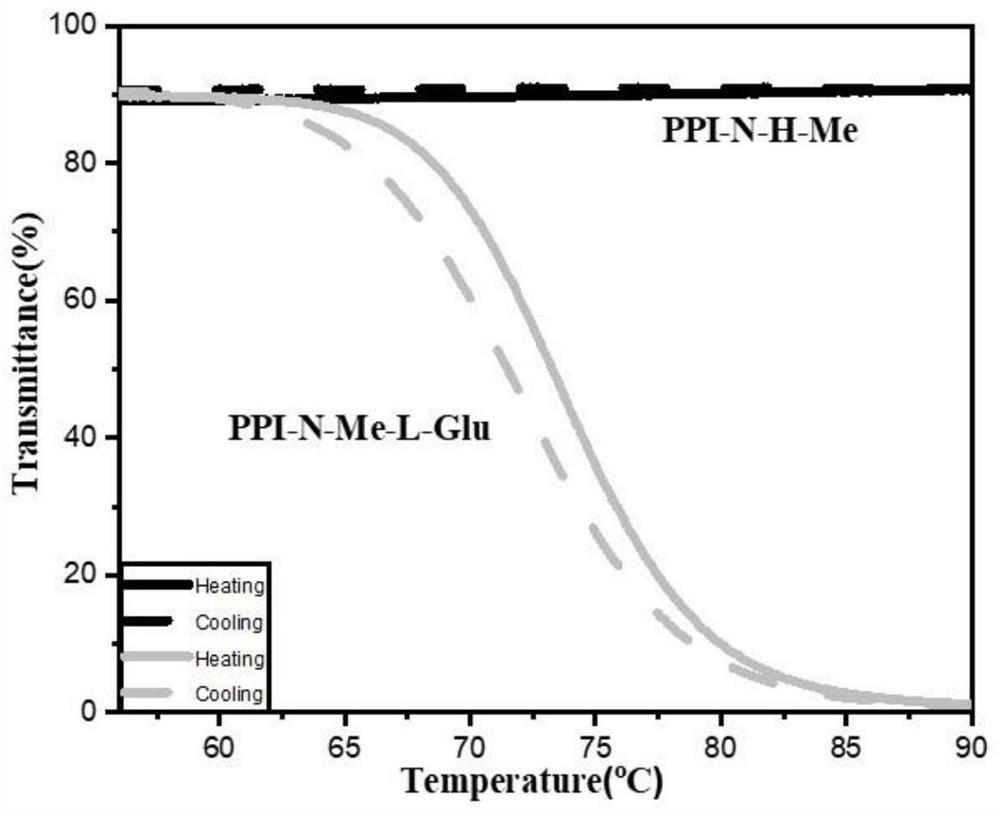
[0050] In this example, dynamic acylhydrazone bonds are used to construct temperature-sensitive helical polyisocyanate derivatives, and small molecules of glutamic acid, alanine, phenylalanine, and leucine are used as chiral units. Induces a main-chain helical conformation. Or adjust the helical conformation of the polyisocyanate main chain by adjusting the temperature. The chirality of small molecules is transferred to the polyisocyanate main chain through dynamic acylhydrazone bonds, and the polyisocyanate is induced to form a helical structure through temperature-sensitive behavior. This type of polymer is suitable for applications in the fields of chiral compound separation and asymmetric catalysis.
[0051] In summary, the present invention utilizes the general-soldier principle to induce the helical conformation of the main chain with a small amount of chiral glutamic acid or other small...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com