Bracket with drug temperature-sensitive controlled-release function and application thereof
A temperature-sensitive, functional technology, applied in the field of stents, to achieve the effect of inhibiting proliferation, good temperature sensitivity, and good drug release function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Preparation of amphiphilic block copolymer of temperature-sensitive polymer and degradable hydrophobic polymer
[0037] (1) Mix NIPAm and NHMAAm at a ratio of 7mmol:1mmol, dissolve them in 10ml tetrahydrofuran (THF), then add 0.04mmol benzoyl peroxide (BPO) to the solution as initiator, 0.08mmol mercaptoethanol for chain transfer Agent, solution pass N 2 Breathe 20min;
[0038] (2) Then in N 2 Placed in a 70℃ constant temperature water bath under the protection of, and react with electromagnetic stirring for 7 hours;
[0039] (3) After the reaction liquid is cooled, suction filtration to remove impurities;
[0040] (4) 8-10 times the volume of ether is used as a precipitant, and white powder is obtained after repeated dissolution, precipitation, and vacuum drying.
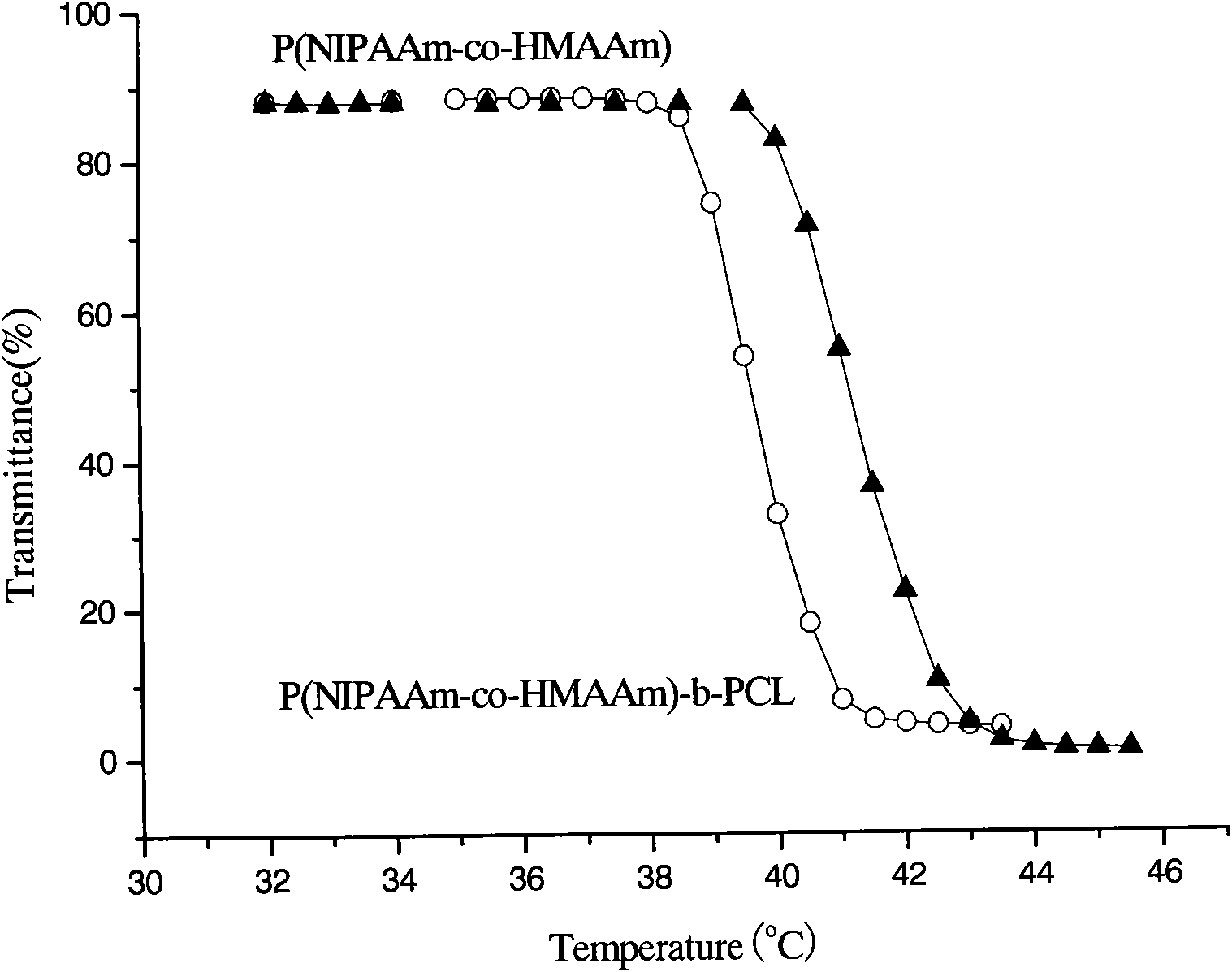
[0041] (5) Copolymerize the prepared methylol acrylamide and N-isopropyl acrylamide with polycaprolactone (PCL) in a ratio of 1:4 (molar ratio), react at 120°C for 24 hours to obtain the desired amphiphilic temper...
Embodiment 2
[0051] 1. Preparation of amphiphilic block copolymer of temperature-sensitive polymer and degradable hydrophobic polymer
[0052] (1) Mix NIPAm and NHMAAm in a ratio of 5mmol:1mmol, dissolve them in 10ml tetrahydrofuran (THF), then add 0.04mmol benzoyl peroxide (BPO) to the solution as the initiator, and 0.10mmol mercaptoethanol for chain transfer Agent, solution pass N 2 Breathe 20min;
[0053] (2) Then in N 2 Placed in a 70℃ constant temperature water bath under the protection of, and react with electromagnetic stirring for 7 hours;
[0054] (3) After the reaction liquid is cooled, suction filtration to remove impurities;
[0055] (4) 8-10 times the volume of ether is used as a precipitant, and white powder is obtained after repeated dissolution, precipitation, and vacuum drying.
[0056] (5) The prepared copolymer and polycaprolactone (PCL) are in a ratio of 1:6 (molar ratio) and reacted at 180°C for 24 hours to obtain the desired amphiphilic temperature-sensitive copolymer P-(NIPAA...
Embodiment 3
[0064] 1. Preparation of amphiphilic block copolymer of temperature-sensitive polymer and degradable hydrophobic polymer
[0065] The specific method is the same as in Example 1, wherein the ratio of poly-N-isopropylacrylamide and polylactide (PLA) is 1:1 (molar ratio).
[0066] 2. Preparation of the coating with temperature-sensitive and controlled-release drug
[0067] Weigh 100 mg of polylactide (PLA) to prepare an organic solution of tetrahydrofuran with a concentration of 10-15% by weight. Weigh camptothecin at a drug / polylactide ratio of 1:20-1:30. Place in the above solution, mix and stir. Weigh the amphiphilic block copolymer at a ratio of 1:1-1:15 amphiphilic block copolymer / polylactide. Place the amphiphilic block copolymer in the above solution and mix, stir and swirl to form a uniform mixed solution .
[0068] 3. Preparation of a stent with drug temperature-sensitive and controlled-release effect
[0069] A tracheal stent (MTN tracheal stent, nickel-titanium alloy materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com