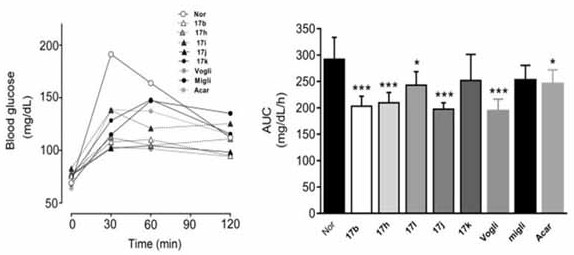
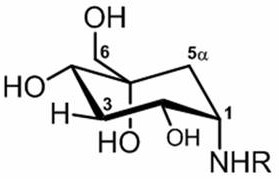
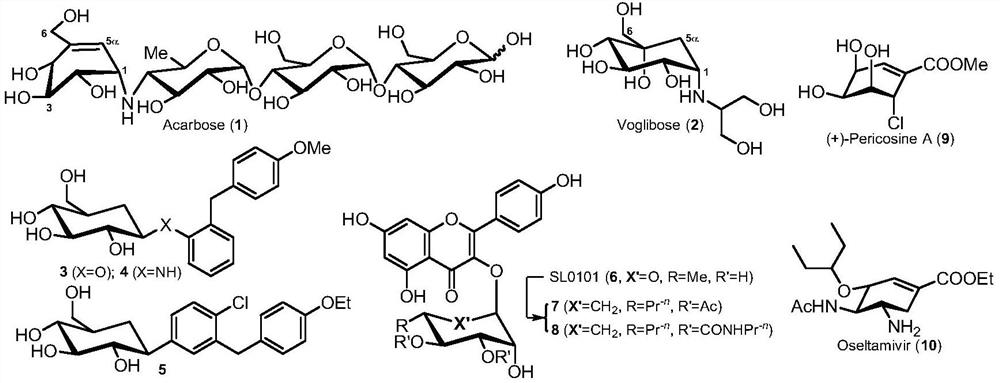
3-deoxy-5-hydroxyl-1-amino carbon sugar compound and its application
A technology of amino carbon sugars and compounds, applied in the preparation of amino hydroxyl compounds, carbon-based compounds, amino sugars, etc., to achieve obvious hypoglycemic activity and significant α-glucosidase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0032] Embodiment 1a: under ice-water bath, compound 14 counts 4.88g (11.0mmol), 10%Pd / C 0.20g, Na 2 CO 3 0.24g and MeOH 100mL were vigorously stirred for atmospheric catalytic hydrogenation for 2.0h. Filtration, the filtrate was washed with 1.0M NaH 2 PO 4 / Na 2 HPO 4 The buffer solution was diluted with 100 mL, and concentrated under reduced pressure to recover methanol. The residue was extracted with ethyl acetate and dried over anhydrous sodium sulfate. After concentration, the crude product was separated by column chromatography to obtain 3.04 g of white solid 12 with a yield of 62.0%. m.p 104~106℃,[α] D20 =+82.88(c 1.0, CHCl 3 ).
[0033] 1 H NMR (600MHz, CDCl 3 ):δ7.29–7.17(m,15H),4.80(d,J=11.9Hz,1H),4.57(d,J=11.5Hz,1H),4.41–4.38(m,4H),3.94(dd, J=11.6Hz, 4.7Hz, 1H), 3.83(dd, J=12.7Hz, 6.1Hz, 1H), 3.56(d, J=8.7Hz, 1H), 3.10(d, J=8.7Hz, 1H), 2.65(d,J=14.7,1H),2.42-2.39(m,1H),2.36(d,J=14.6,1H),2.07(q,J=12.2Hz,1H).
[0034] 13 C NMR (150MHz, CDCl 3 ): δ206.1...
Embodiment 1b
[0036] Example 1b: Under similar conditions, 10% Ru / C 0.25g was used instead of Pd / C in Example 1a, and 2.89g of 12 was obtained after similar post-treatment, with a yield of 59.0%.
Embodiment 1c
[0037] Example 1c: Under similar conditions, 0.40 g of Raney / Ni(W–2) was used instead of Pd / C in Example 1a. After similar post-treatment, 2.40 g of 12 was obtained, with a yield of 49.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com