Synthetic method of diethanol nitramine dinitrate
A technology of diethanol nitramine dinitrate and diethanolamine hydrochloride, applied in the direction of organic chemistry and the like, can solve problems such as high explosion risk, and achieve the effects of improving reaction efficiency, easy control of synthesis process and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic method of the diethanol nitramine dinitrate of present embodiment 1 comprises the following steps:
[0030] Add 345g of acetic anhydride containing a small amount of 40g of fuming nitric acid to the 1L reaction calorimeter RC1e reactor, adjust the temperature of the reactor to 10°C, and then add 137g of diethanolamine hydrochloride and 195g of fuming nitric acid at the same time. The molar ratio of diethanolamine hydrochloride to fuming nitric acid to acetic anhydride is 1:3.2:3.5. After the feeding is finished, wait for 30 minutes, raise the temperature of the system to 40°C, and keep the temperature for 30 minutes. After completion, the reaction solution was poured into ice water to precipitate crystals, and the target product diethanolamine dinitrate 1 was obtained by suction filtration and drying. Yield 72.5%.
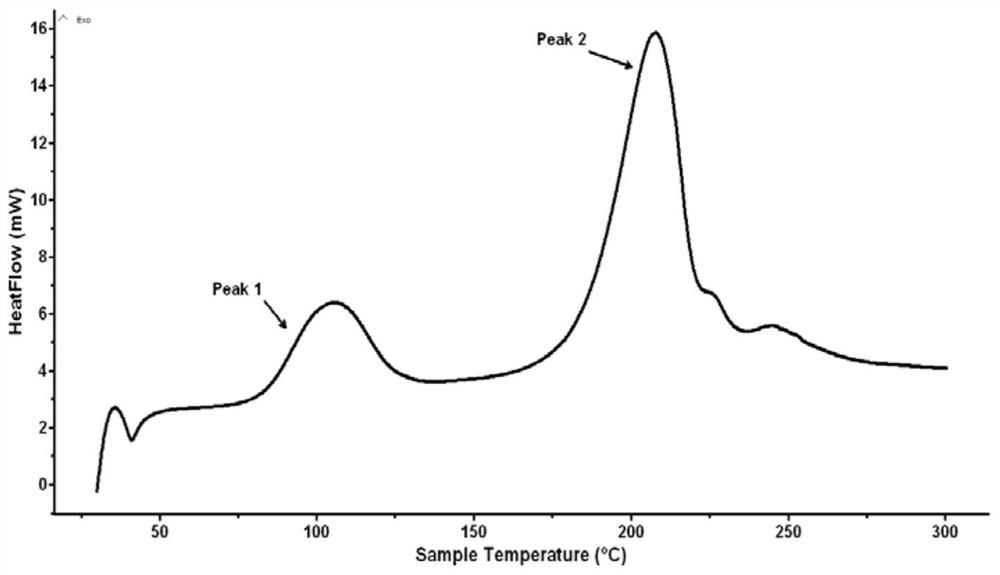
[0031] Due to incomplete nitration, the product contains impurities and is not pure. Carry out DSC test and nuclear magnetic characterizati...
Embodiment 2
[0033] The synthetic method of the diethanol nitramine dinitrate of present embodiment 2 comprises the following steps:
[0034] Add 345g of acetic anhydride containing a small amount of 40g of fuming nitric acid to the 1L reaction calorimeter RC1e reactor, adjust the temperature of the reactor to 10°C, and then add 100g of diethanolamine hydrochloride and 200g of fuming nitric acid at the same time. The molar ratio of diethanolamine hydrochloride to fuming nitric acid to acetic anhydride is 1:4.5:4.8. After the feeding was finished, because the amount of solvent was too small, crystallization occurred immediately in the reactor, and the target product diethanolamine dinitrate 2 was separated out, and the experiment ended. The yield is 73.8%.
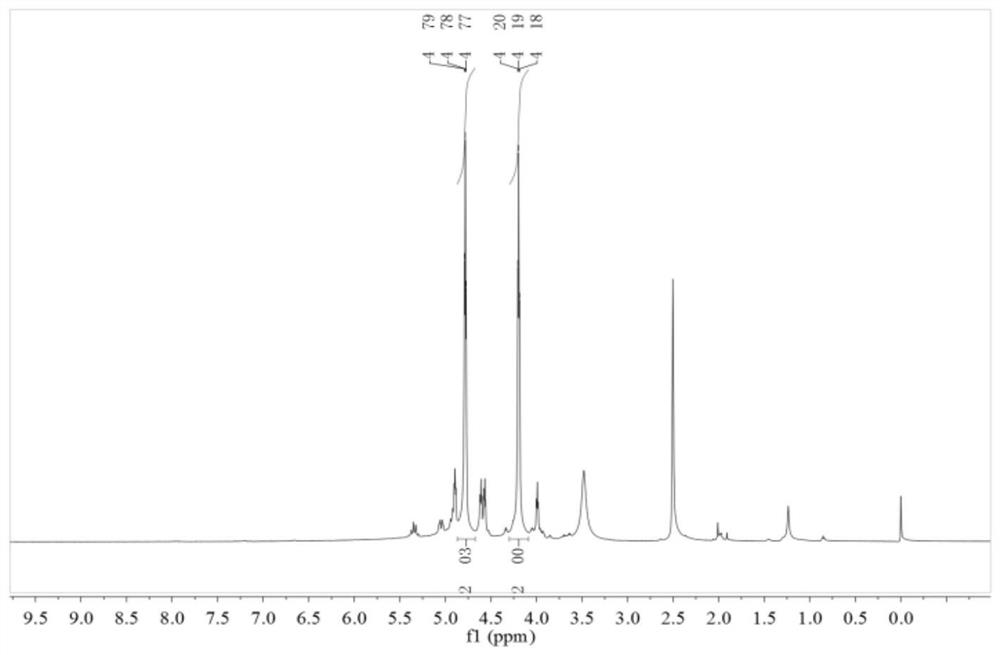
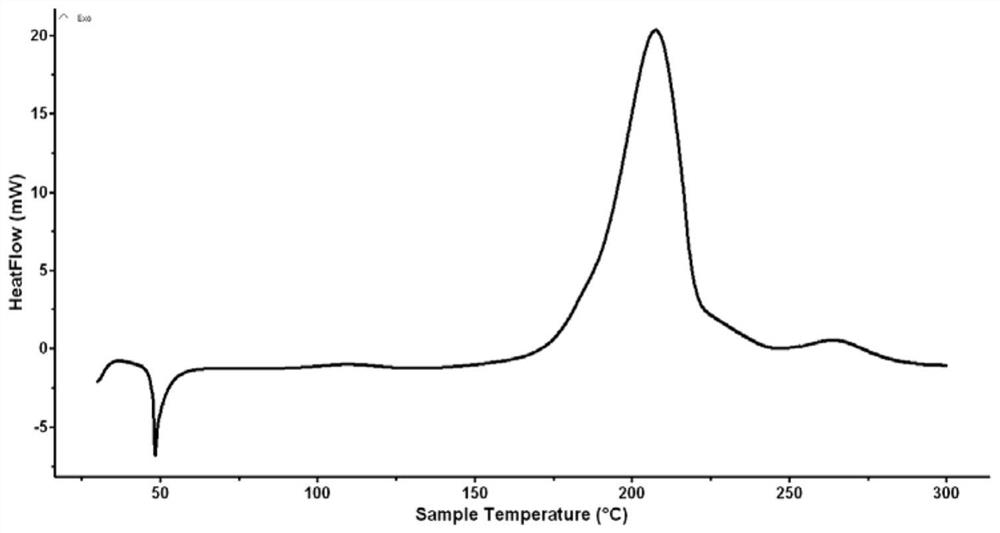
[0035] There were no impurities in the product. Carry out DSC test and nuclear magnetic characterization to the product, the result sees image 3 , 4 shown.
Embodiment 3
[0037] The synthetic method of the diethanol nitramine dinitrate of present embodiment 3 comprises the following steps:
[0038] Add 345g of acetic anhydride containing a small amount of 45g of fuming nitric acid to the 1L reaction calorimeter RC1e reactor, adjust the temperature of the reactor to 10°C, and then simultaneously add 80g of diethanolamine hydrochloride and 150g of fuming nitric acid. The molar ratio of diethanolamine hydrochloride to fuming nitric acid to acetic anhydride is 1:4.5:6. After the feeding is finished, wait for 60 minutes, raise the temperature of the system to 40°C, and keep the temperature for 30 minutes. After completion, the reaction solution was poured into ice water to precipitate crystals, and the target product diethanolamine dinitrate 3 was obtained by suction filtration and drying. Yield 82%.
[0039] There were no impurities in the product. The heat release rate q of the reaction process r , Reaction temperature T r curve see Figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com