Preparation method of ultrafine amorphous boron powder
A technology of amorphous boron powder and boron powder, which is applied in the direction of boron, boron/boride, etc., can solve the problems of pollution, average particle size, and discontinuous production cannot be further developed, so as to reduce costs, The effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
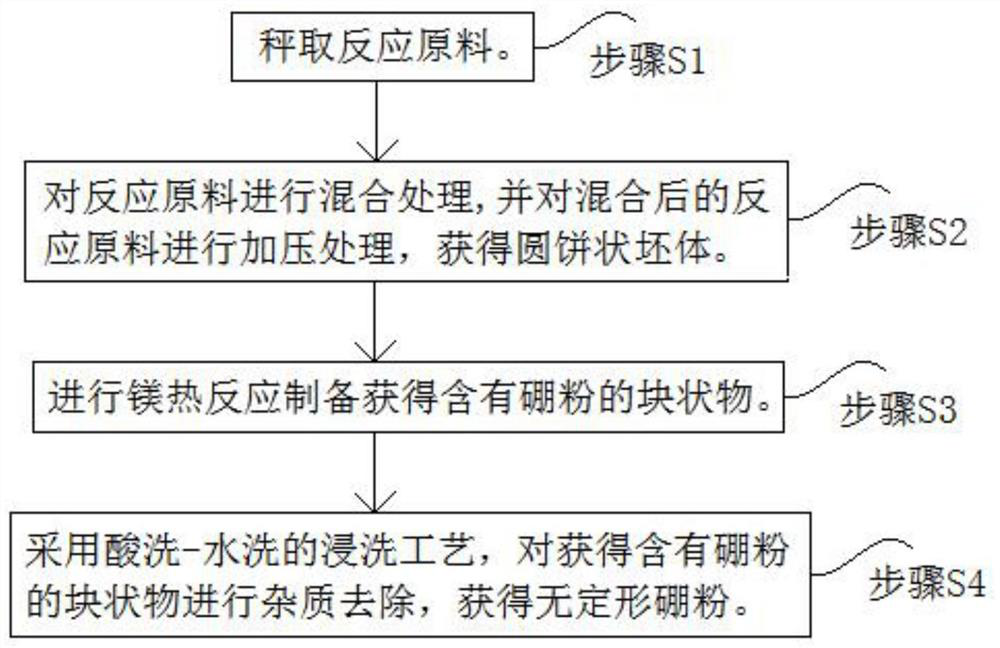
[0022] combine figure 1 Shown, the concrete process of the superfine amorphous boron powder of preparation high purity, low particle size is as follows:
[0023] Step S1, weighing the reaction raw materials. In terms of mass percentage, the reaction raw materials include Mg powder 41.65%, B 2 o 3 Powder 36.15%, diluent powder 22.20%, wherein the diluent powder is KCl powder. In addition, it is required that the Mg powder should reach more than 95% of industrial purity, and B 2 o 3 The powder reaches more than 95% of analytical purity and the KCl powder reaches more than 99.5% of analytical purity.
[0024] In step S2, the reaction raw materials are mixed, and the mixed reaction raw materials are pressurized to obtain a round cake-shaped body.
[0025] In this embodiment, firstly, the reaction raw materials are mixed by means of a QM-1SP4 planetary ball mill. Among them, the speed of the ball mill is set to 150r / min, the ratio of ball to material is set to 2:1, and the m...
Embodiment 2
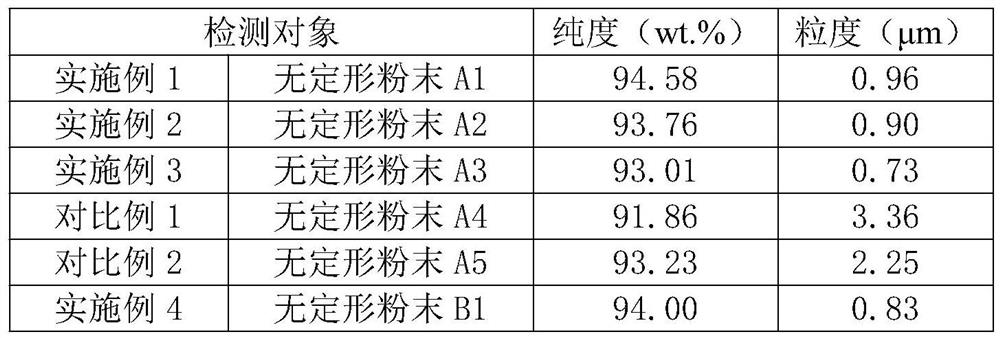
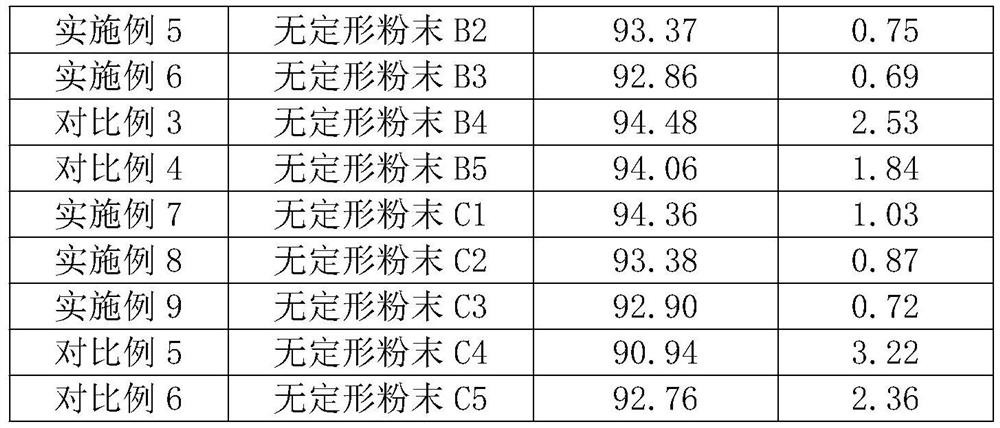
[0033] Adopt the same method as Example 1 to prepare amorphous boron powder, the only difference is that in step S1, the mass percentage of Mg powder in the mixed powder for preparing amorphous boron powder is 38.78%, B 2 o 3 The mass percentage of the powder is 33.66%, the KCl powder is 27.56%, and the amorphous boron powder marked A2 is obtained.
Embodiment 3
[0035] Adopt the same method as Example 1 to prepare amorphous boron powder, the only difference is that in step S1, the mass percentage of Mg powder in the mixed powder for preparing amorphous boron powder is 36.28%, B 2 o 3 The mass percentage of the powder is 31.49%, the KCl powder is 32.23%, and the amorphous boron powder marked A3 is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com