Aryl oxazolidinone compound of P-difluoroalkyl and preparation method thereof
A compound and alkyl technology, applied in the field of difluoroalkyl aryl oxazolidinone compounds and their preparation, can solve the problems of many steps, poor compatibility of functional groups, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
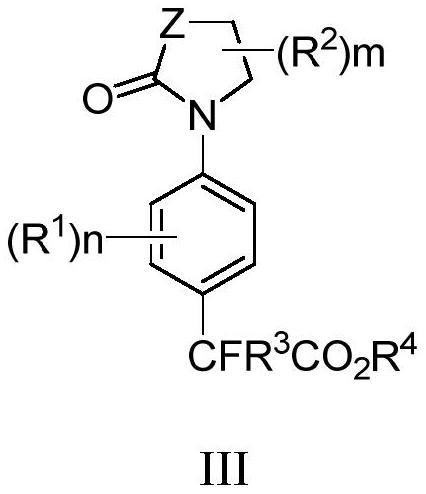
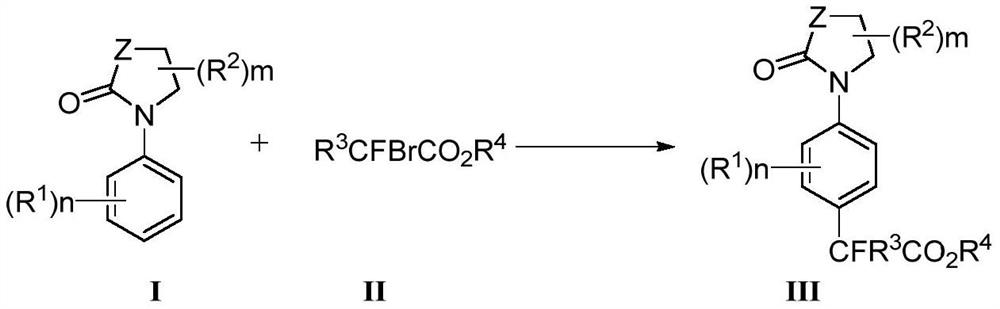
[0097] Example 1 Synthesis of aryl oxazolidinones to difluoroalkyl
[0098]
[0099] where R 1 , R 2 The number of substituents is not limited.
[0100] Mix 0.25mmol 3-aryloxazolidinone substrate 1 and 4equiv ethyl difluorobromoacetate 2, add 10mol% catalyst, 20mol% ligand and 2equiv base to the reaction tube, add dry n-Hexane (1mL), react at 140°C for 24h to obtain a mixed liquid, add water and ethyl acetate to extract three times after the reaction, combine the organic layers, anhydrous Na 2 SO 4 The organic layer was dried, filtered, concentrated, separated and purified by silica gel column chromatography (petroleum ether / ethyl acetate=50 / 1-1 / 1) to obtain para-difluoroalkylated aryl oxazolidinone compound 3.
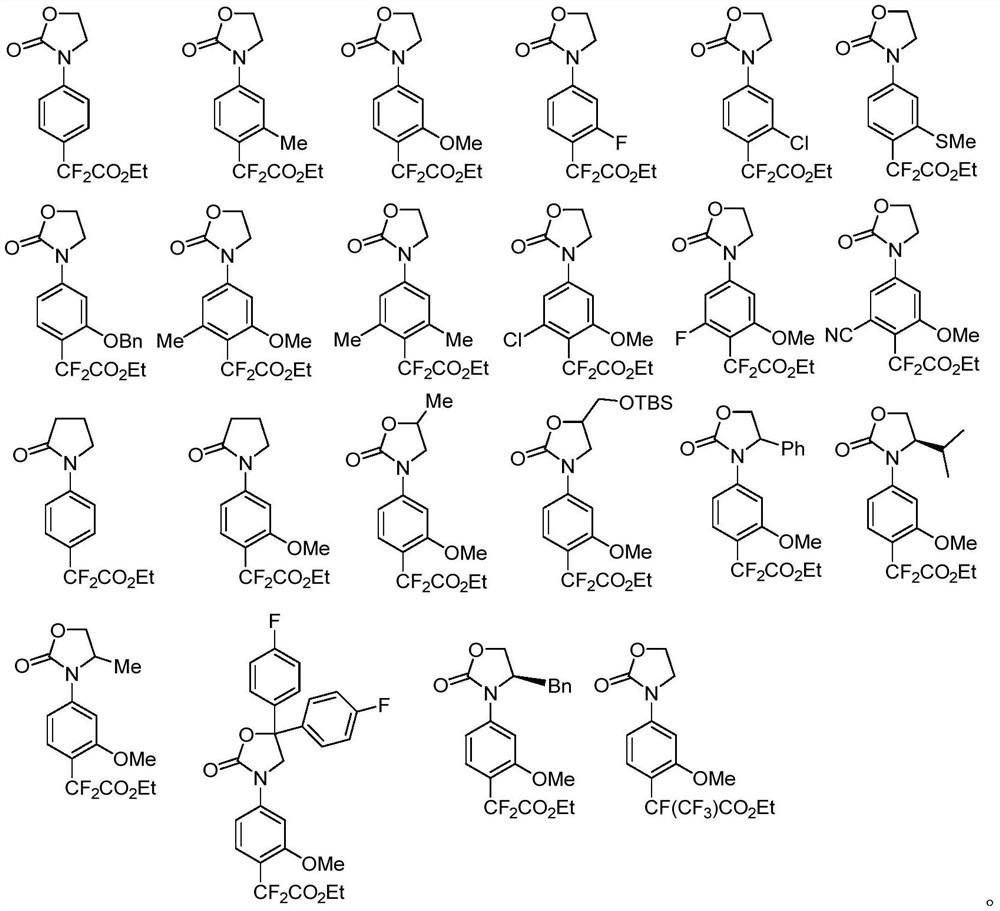
[0101] According to the above method, according to the specific structural formula of the para-difluoroalkylated aryl oxazolidinone compound to be synthesized, select the corresponding aryl oxazolidinone substrate and ethyl difluorobromoacetate to react, Such as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com