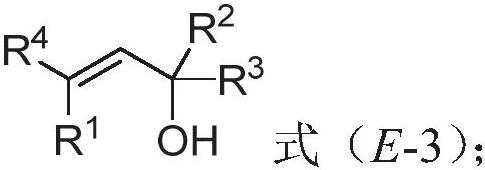
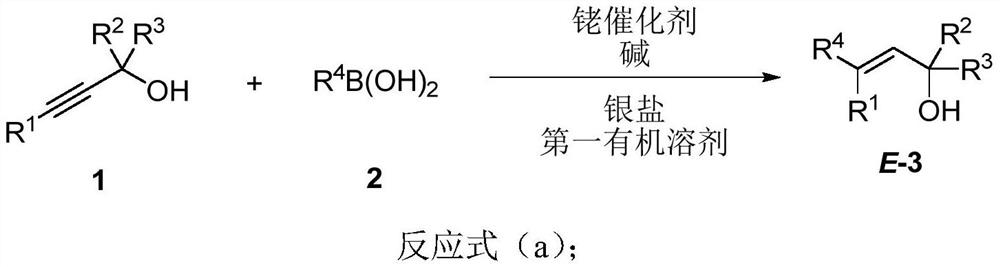
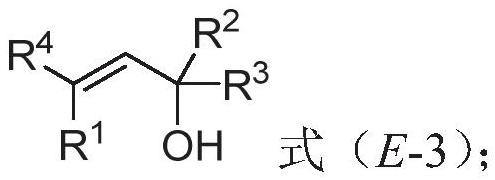
Polysubstituted beta-phenylallyl alcohol compound as well as synthesis method and application thereof
A phenylpropenyl alcohol, multi-substitution technology, which is applied to the effective components of hydroxyl compounds, carbon-based compound preparation, chemical instruments and methods, etc., can solve the problems such as the activity of multi-substituted β-phenylpropenyl alcohol compounds that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083]
[0084] The dry reaction tube was wrapped in aluminum foil to avoid light, and AgBF was added in the glove box 4 (29.8mg, 0.15mmol), after removing from the glove box, add [Cp*RhCl 2 ] 2 (15.5 mg, 0.025 mmol), NaOAc (16.7 mg, 0.20 mmol), 3-methoxyphenylboronic acid (2a, 228.5 mg, 1.5 mmol), 1a (139.7 mg, 1.0 mmol), MeOH (5 mL). The reaction tube is filled with CaCl 2 Open the drying tube to the atmosphere and stir at room temperature for 40 minutes. The reaction solution was filtered through a diatomaceous earth short column (3 cm), washed with MeOH, and the solvent was removed by rotary evaporation. Separation and purification were carried out by silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 10 / 1) to obtain product E-3aa (211.1 mg, 85%): pale yellow liquid; 1 H NMR (400MHz, CDCl 3 )δ=7.22(t, J=8.0Hz, 1H, ArH), 6.89(d, J=8.0Hz, 1H, ArH), 6.86-6.82(m, 1H, ArH), 6.79(dd, J 1 =7.8Hz,J 2 =2.6Hz, 1H, ArH), 5.70(s, 1H, =CH), 3.81(s, 3H, ...
Embodiment 2
[0086]
[0087] The dry reaction tube was wrapped in aluminum foil to avoid light, and AgBF was added in the glove box 4 (29.5mg, 0.15mmol), after removing from the glove box, add [Cp*RhCl 2 ] 2 (15.8 mg, 0.025 mmol), NaOAc (16.5 mg, 0.20 mmol), 4-formylphenylboronic acid (2b, 229.6 mg, 1.5 mmol), 1a (140.6 mg, 1.0 mmol), MeOH (5 mL), at room temperature Stir for 4 hours. The reaction solution was filtered through a diatomaceous earth short column (3 cm), washed with MeOH, and the solvent was removed by rotary evaporation. Separation and purification were carried out by silica gel column chromatography (eluent: separation by column chromatography for the first time: petroleum ether, petroleum ether / ethyl acetate=30 / 1-10 / 1, to obtain 94.6 mg of pure product and 109.4 mg of impure product ; The impure product was separated by column chromatography for the second time: dichloromethane, to obtain 91.7 mg of pure product), to obtain product E-3ab (186.3 mg, 75%): yellow liqui...
Embodiment 3
[0089]
[0090] Operation is with embodiment 1. ikB 4 (29.0mg, 0.15mmol), [Cp*RhCl 2 ] 2 (15.8 mg, 0.025 mmol), NaOAc (17.3 mg, 0.20 mmol), 4-fluorophenylboronic acid (2c, 210.3 mg, 1.5 mmol), 1a (139.4 mg, 1.0 mmol), MeOH (5 mL), reacted for 19 hours to obtain E-3ac (130.7 mg, 56%) (crude spectrum shows 15% of raw material remaining) (eluent: toluene): pale yellow liquid; 1 H NMR (400MHz, CDCl 3 )δ=7.31-7.20(m, 2H, ArH), 7.04-6.92(m, 2H, ArH), 5.64(s, 1H, =CH), 2.78(t, J=7.6Hz, 2H, CH 2 ), 1.61(brs, 1H, OH), 1.46(s, 6H, 2x CH 3 ), 1.36-1.18 (m, 4H, 2x CH 2 ), 0.84(t, J=7.0Hz, 3H, CH 3 ); 13 C NMR (100MHz, CDCl 3 ,) δ = 161.9 (d, J = 243.6Hz), 142.1, 139.7 (d, J = 3.0Hz), 135.8, 128.1 (d, J = 7.6Hz), 114.9 (d, J = 20.5Hz), 71.3, 31.6, 31.0, 29.8, 22.9, 13.9; 19 F NMR (376MHz, CDCl 3 )δ=-116.8; IR (neat): v=3357, 2961, 2928, 2861, 1637, 1601, 1506, 1465, 1362, 1220, 1157, 1106, 1014cm -1 ; MS (70eV, EI) m / z (%): 236 (M + , 4.74), 109(100); HRMS calcd for C 15 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com