Pharmaceutical composition containing aryl propionic acid compound
A technology of compounds and compositions, applied in the field of medicinal chemistry, can solve problems such as inability to meet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 formula I compound
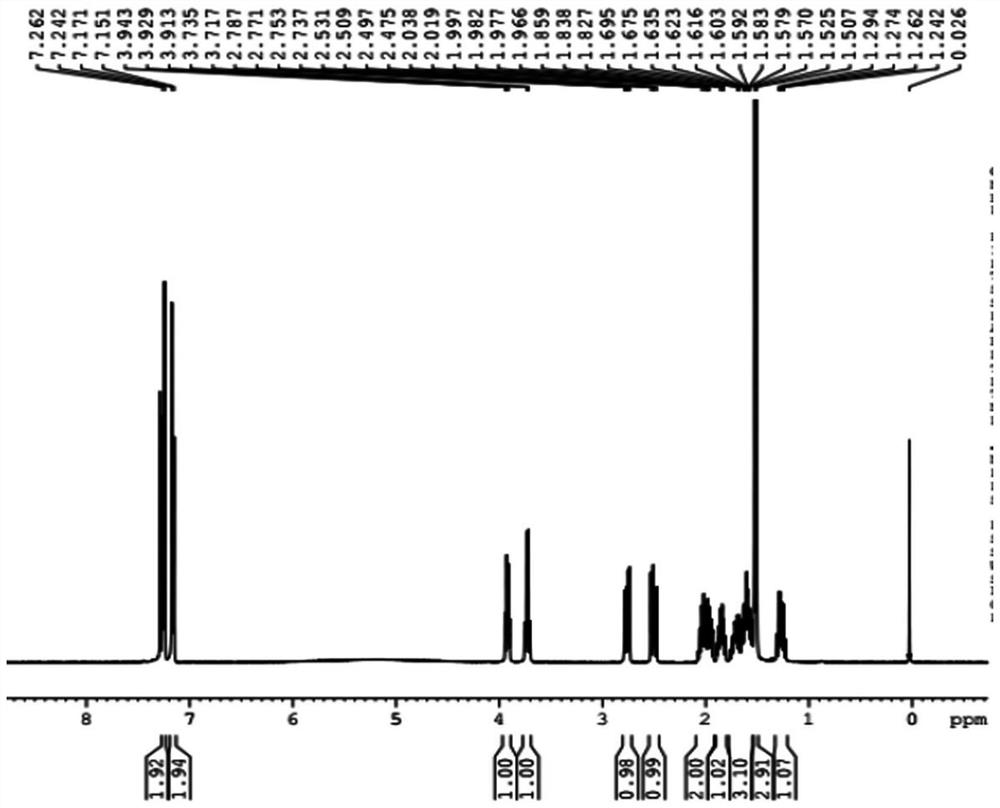
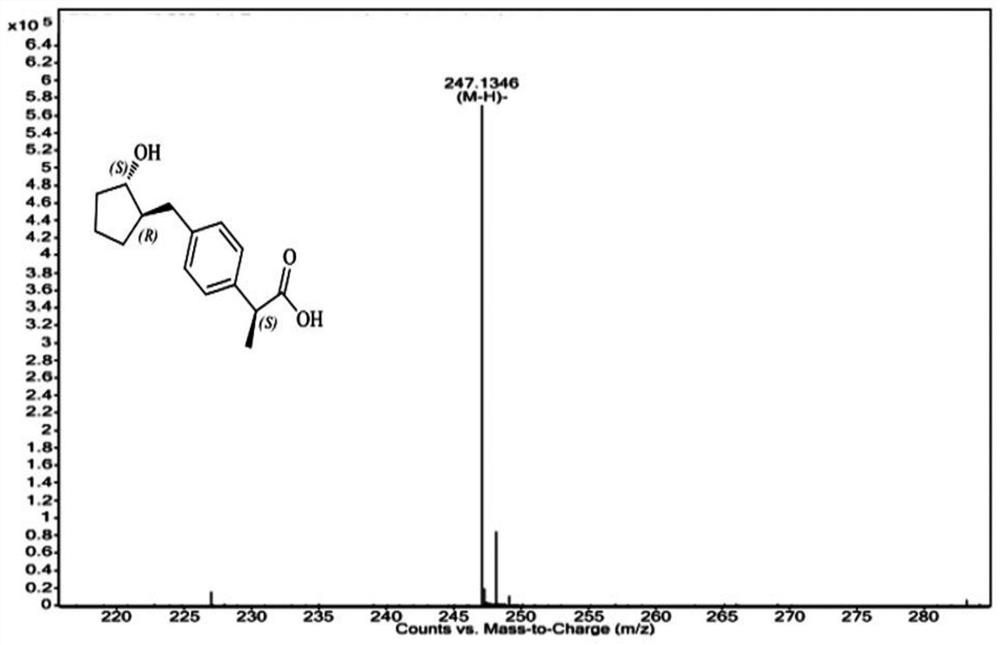
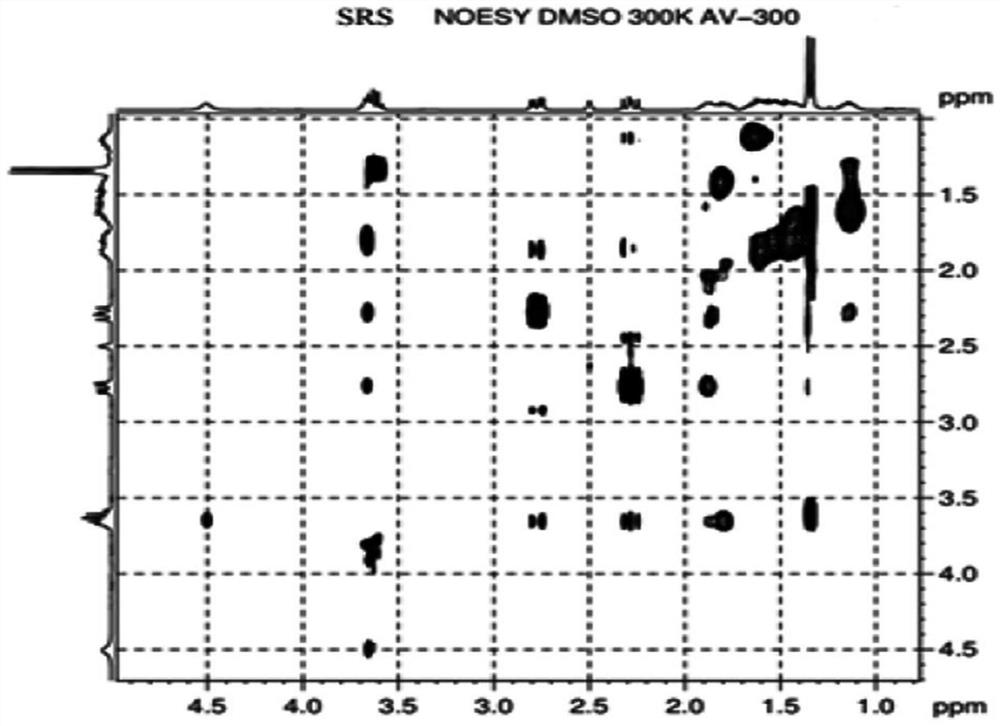
[0041] The preparation method of the compound of formula I is obtained from the reference "Tetrahedron Asymmetry, 2011, 22: 1125-1132". Those of ordinary skill in the art can obtain this compound through conventional synthesis methods, and the structure has been confirmed, see the attached Figure 1-3 ,No longer.
[0042] It is worth noting that, in the prescriptions of the following preparation examples, the selection and dosage of each component of the main drug and auxiliary materials are only for illustration. Those skilled in the art can make conventional adjustments, replacements and changes based on information such as therapeutic doses, specifications, and administration characteristics. degree. These should also belong to the scope of the design idea of the present invention. In addition, in the preparation examples of the present invention, the dosage of the main drug is based on the weight of the acti...
Embodiment 2
[0043] Example 2 Preparation of Formula I Compound Injection
[0044] Main drug 10g
[0047] Water for injection to 1000mL;
[0048] Preparation method: Add water for injection with 80% of the prescription amount in the preparation container, weigh the main drug, sodium hydroxide and sodium chloride, dissolve and stir evenly, adjust the pH to 6.9 with sodium hydroxide and / or hydrochloric acid solution, and dilute to Final volume; filter with a 0.22 μm pore size sterile filter, divide the filtered solution into ampoules, seal, and sterilize at 121°C for 20 minutes to obtain the final product.
Embodiment 3
[0049] Example 3 Preparation of Formula I Compound Injection
[0050] Main ingredient 10g
[0051] L-Arginine 7g
[0052] Sodium chloride 8g
[0053] Water for injection to 1000mL
[0054] Preparation method: Add water for injection with 80% of the prescription amount in the preparation container, weigh the main drug, L-arginine and sodium chloride to dissolve, stir evenly, adjust the pH to 7.2 with arginine solution, and dilute to the final volume ; Use a 0.22 μm pore size sterile filter to filter, the filtered solution is divided into ampoules, sealed, and sterilized at 121 ° C for 20 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com