Method for synthesizing beta-thiocarbonyl compound by taking aryl sulfonyl chloride as sulfur source
A technology of arylsulfonyl chloride and thiocarbonyl, which is applied in the field of synthesizing β-thiocarbonyl compounds, and can solve problems such as unfavorable practical production, easy volatilization, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
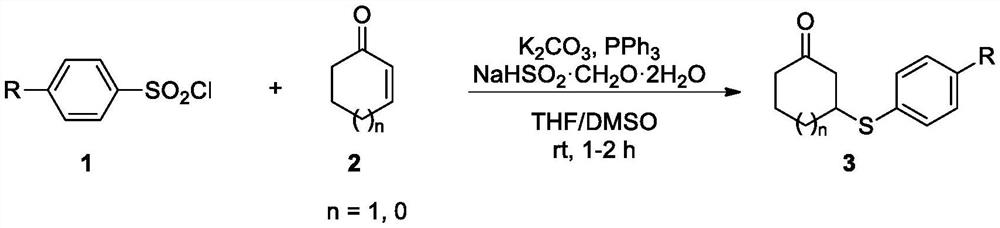
[0040] In the air atmosphere, add 0.3mmol of p-toluenesulfonyl chloride, 0.6mmol of triphenylphosphine, 0.3mmol of potassium carbonate, 0.6mmol of sodium bisulfite formaldehyde, 0.3mmol of 2-cyclohexenone, and add 3mL of mixed solvent ( THF:DMSO=1:1; volume ratio), magnetically stirred at 25-50° C. for 1-2 h, and monitored by TLC during the reaction until complete reaction. After-treatment, an appropriate amount of ethyl acetate was added for extraction, dried over anhydrous sodium sulfate, and the pure 3a was separated by column chromatography after spin-drying the solvent under reduced pressure. Isolated yield: 82%.
[0041] 1 H NMR (CDCl 3 ,400MHz)δ7.32(d,J=8.0,2H),7.11(d,J=8.0,2H),3.33-3.32(m,1H),2.66-2.62(m,1H),2.38-2.28(m ,6H),2.12-2.09(m,2H),1.71-1.68(m,2H); 13 C NMR (CDCl 3 ,100MHz)δ208.99,138.26,134.08,129.96,129.28,47.92,46.60,41.00,31.40,24.19,21.26.
Embodiment 2
[0043]
[0044] In air atmosphere, add 0.3mmol of benzenesulfonyl chloride, 0.6mmol of triphenylphosphine, 0.3mmol of potassium carbonate, 0.6mmol of sodium bisulfite formaldehyde, 0.3mmol of 2-cyclohexenone, and add 3mL of mixed solvent (THF :DMSO=1:1; volume ratio), magnetically stirred at 25-50°C for 1-2h, and monitored by TLC during the reaction until complete reaction. After-treatment, an appropriate amount of ethyl acetate was added for extraction, dried over anhydrous sodium sulfate, and the pure 3b was separated by column chromatography after spin-drying the solvent under reduced pressure. Isolated yield: 81%.
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.43-7.40(m,2H),7.33-7.25(m,3H),3.45-3.40(m,1H),2.70-2.65(m,1H),2.40-2.27(m,3H),2.16- 2.10(m,2H),1.78-1.65(m,2H); 13 C NMR (100MHz, CDCl 3 )δ208.6, 133.10, 133.02, 129.04, 127.61, 47.72, 46.03, 40.82, 31.12, 24.03.
Embodiment 3
[0047]
[0048] Add 0.3mmol p-methoxybenzenesulfonyl chloride, 0.6mmol triphenylphosphine, 0.3mmol potassium carbonate, 0.6mmol sodium bisulfite formaldehyde, 0.3mmol 2-cyclohexenone to 3mL In a mixed solvent (THF:DMSO=1:1; volume ratio), magnetically stirred at 25-50° C. for 1-2 h, and monitored by TLC during the reaction until complete reaction. After-treatment, an appropriate amount of ethyl acetate was added for extraction, dried over anhydrous sodium sulfate, and the pure 3c was separated by column chromatography after spin-drying the solvent under reduced pressure. Isolated yield: 85%.
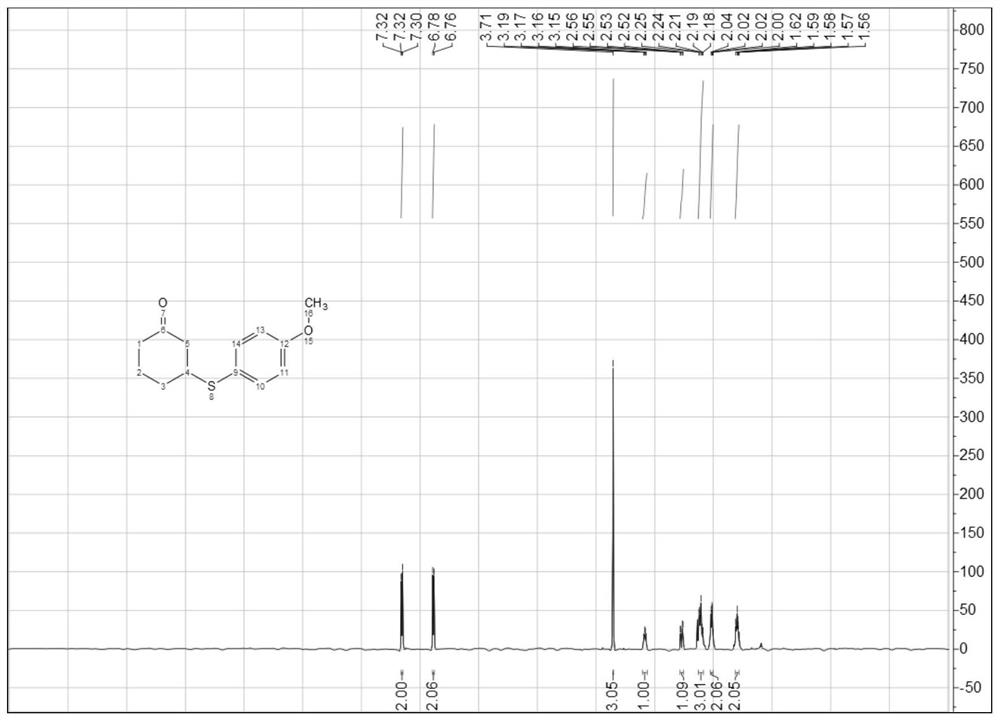
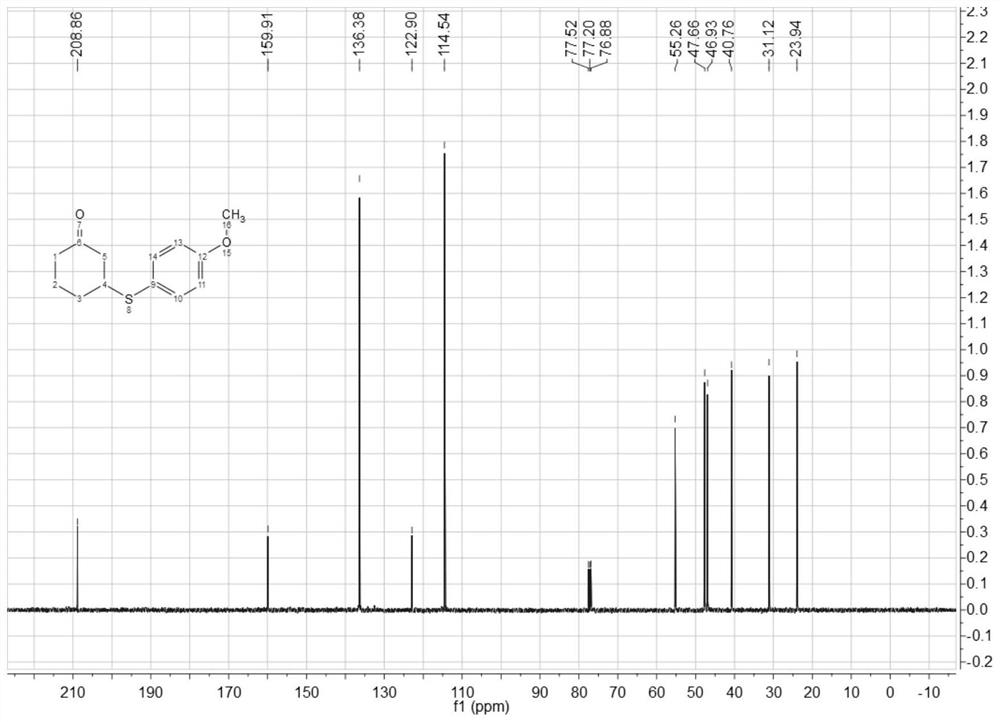
[0049] 1 H NMR (400MHz, CDCl 3 )δ7.32(d, J=8.4Hz, 2H), 6.77(d, J=8.4Hz, 2H), 3.71(s, 3H), 3.17(dd, J=10.5, 7.7Hz, 1H), 2.54( dd,J=14.2,4.2Hz,1H),2.26–2.17(m,3H),2.05–2.01(m,2H),1.59(dd,J=11.5,6.6Hz,2H); 13 C NMR (100MHz, CDCl3) δ208.86, 159.91, 136.38, 122.90, 114.54, 55.26, 47.66, 46.93, 40.76, 31.12, 23.94.
[0050] Example 3 mainly investigates the applicability of substrates ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com