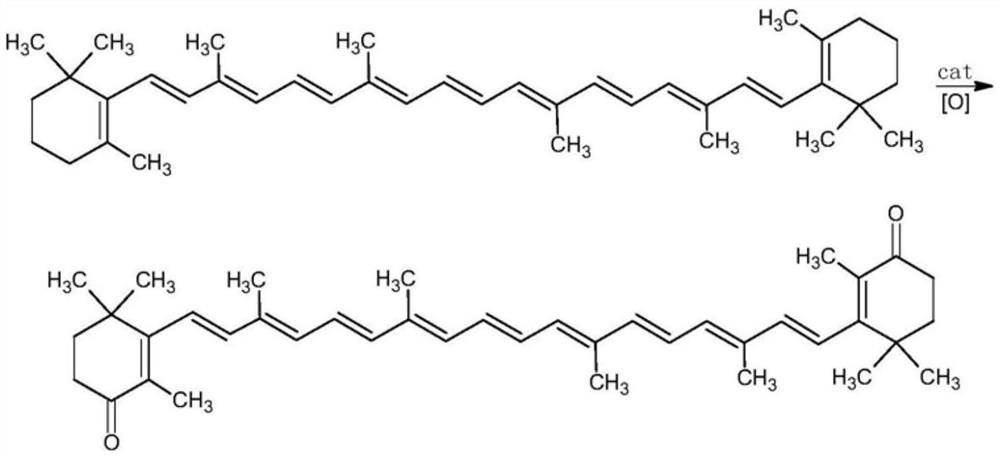
Method for preparing canthaxanthin by oxidizing beta-carotene
A carotene and canthaxanthin technology is applied in the field of canthaxanthin oxidation by β-carotene, which can solve the problems of inconvenient operation, unfriendly environment, and large discharge of three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
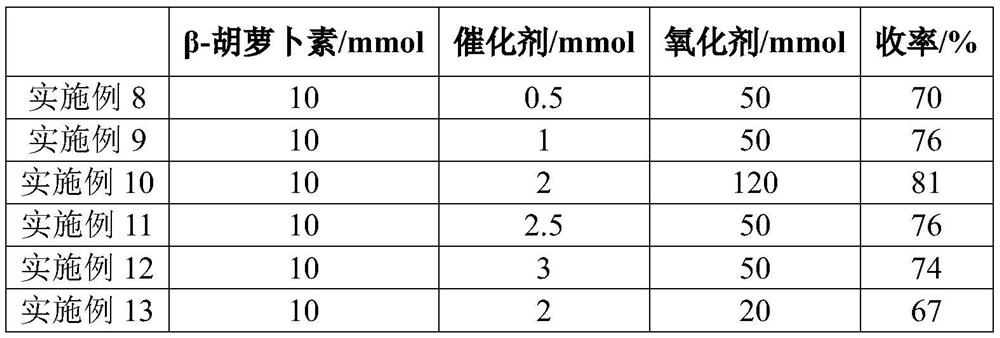
[0027] Put 5.36g (10mmol) of β-carotene in a 500ml three-necked flask, add 100ml of acetonitrile, stir at room temperature, add 0.22g (2.0mmol) of catalyst calcium chloride, and then add 70% of oxidant tert-butanol peroxide. Aqueous solution 5.29g (50mmol), heat up to 70 ° C, reflux reaction, reaction pressure normal pressure, reaction time is 6h, after the reaction is completed, add sodium thiosulfate aqueous solution to quench the reaction, wash with water 3 times, separate the organic phase, and spin dry , and then recrystallized with acetone to obtain 4.55 g of purple canthaxanthin with a yield of 81%.
[0028] 1H NMRδ: 6.65(dt, J=20.4Hz, 7.6Hz, 4H), 6.32(ddd, J=36.4Hz, 27.1Hz, 15.8Hz, 10H), 2.47-2.54(m, 4H), 1.96-2.05(m ,12H), 1.81-1.89(m,10H), 1.19(s,12H).
Embodiment 2
[0030] Put 5.36g (10mmol) of β-carotene into a 500ml three-necked flask, add 100ml of acetonitrile, stir at room temperature, add 0.22g (2.0mmol) of catalyst calcium chloride, and then add 5.67g of 30% aqueous solution of oxidant hydrogen peroxide (50mmol), the temperature was raised to 60°C, the reaction pressure was normal pressure, and the reaction time was 6h. After the reaction was completed, an aqueous solution of sodium thiosulfate was added to quench the reaction, washed with water for 3 times, the organic phase was separated, spin-dried, and then recrystallized with acetone , to obtain purple canthaxanthin 4.06g, yield 72%.
Embodiment 3
[0032] Put 5.36g (10mmol) of β-carotene into a 500ml three-necked flask, add 100ml of acetonitrile, stir at room temperature, add 0.40g (2.0mmol) of catalyst calcium bromide, and then add 70% aqueous solution of oxidant tert-butanol peroxide 5.29g (50mmol), heat up to 90°C, reflux reaction, reaction pressure normal pressure, reaction time is 6h, after the reaction is finished, add sodium thiosulfate aqueous solution to quench the reaction, wash with water 3 times, separate the organic phase, spin dry, Then recrystallized with ethanol to obtain 4.23 g of purple canthaxanthin with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com