A class of naphthocoumarin compounds and preparation methods and their application as photoluminescent materials
A technology of naphthocoumarin and compounds, which is applied in the field of application of naphthocoumarin compounds and photoluminescent materials, and can solve problems such as inability to apply clinical treatment, poor water solubility, and single synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
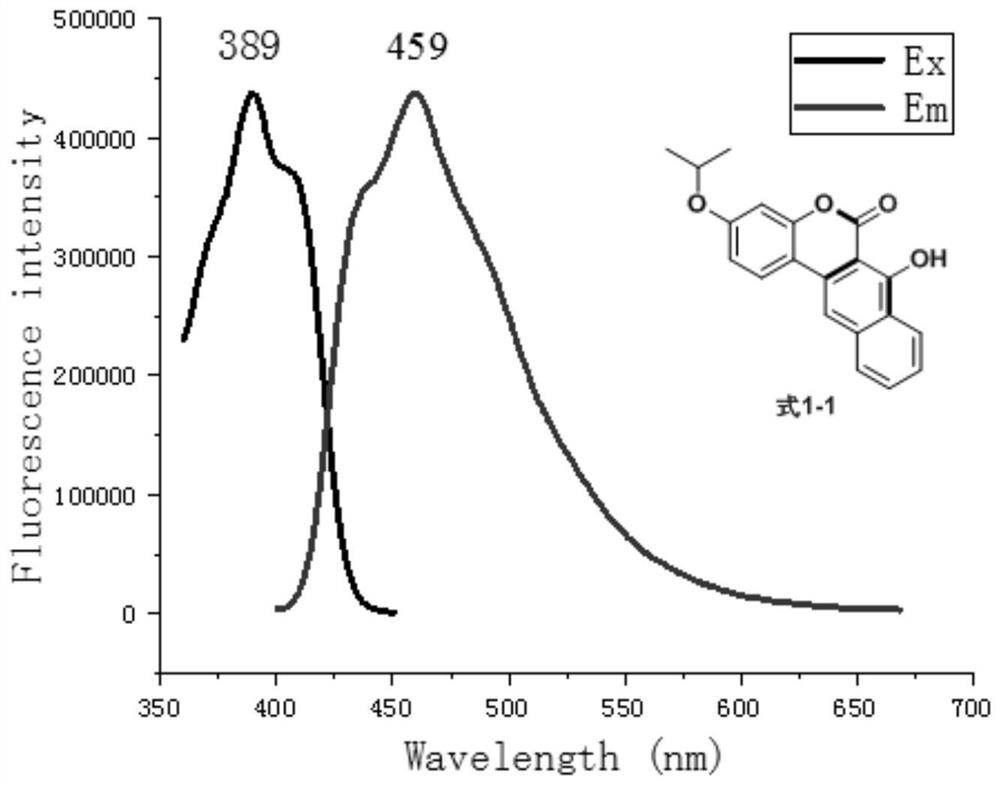
[0036] Prepare formula 1-1 compound according to following reaction equation
[0037]
[0038]Add 1-(2-hydroxyl-4-isopropoxyphenyl)-2-phenyl-1-ethanone (270mg, 1mmol) shown in formula 5-1 and rice shown in formula 6 into a 25mL round bottom flask Acid (288mg, 2mmol), TsOH (35mg, 0.2mmol), 10mL of a mixture of toluene and p-xylene with a volume ratio of 1:1, put on a condenser, and react at reflux at 150°C for 4 hours. Recover the solvent by distillation under reduced pressure. Separation and purification by silica gel column chromatography (the eluent is a mixture of petroleum ether and ethyl acetate with a volume ratio of 50:1 to 10:1) to obtain the compound of formula 1-1: 7-hydroxy-3-isopropoxy-naphthalene And coumarin.
[0039] In the preparation process of the above-mentioned compound of formula 1-1, 1-(2-hydroxyl-4-isopropoxyphenyl)-2-phenyl-1-ethanone shown in formula 5-1 is respectively mixed with equimolar 1 -(2-hydroxyphenyl)-2-phenyl-1-ethanone, 1-(2-hydroxy-5-...
Embodiment 2
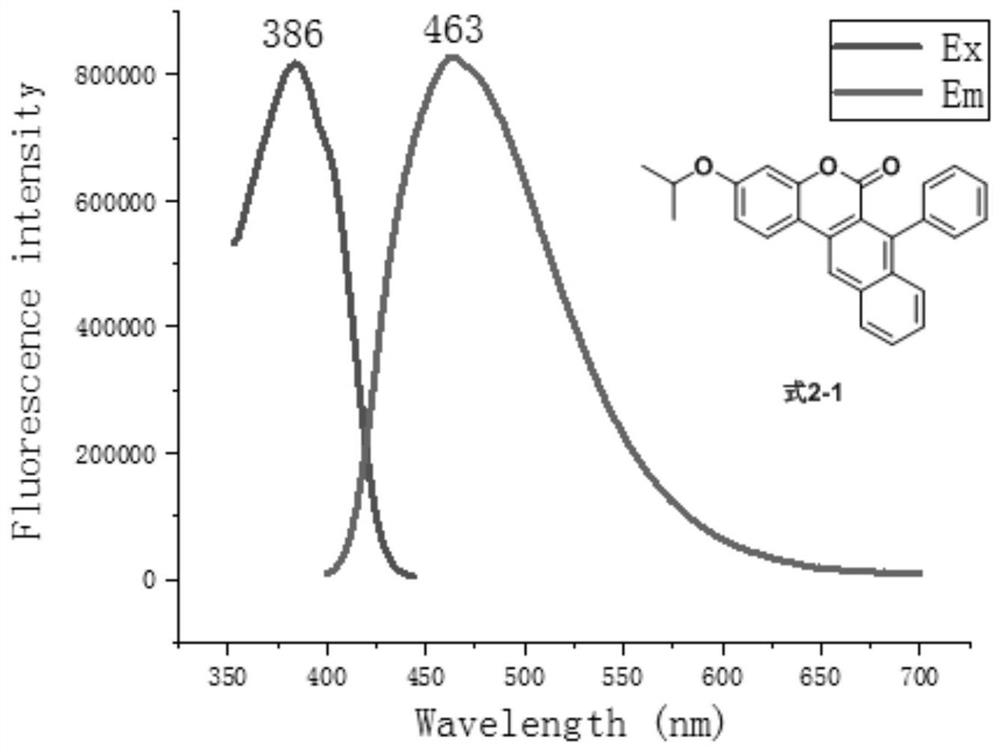
[0059] Compound shown in formula 2-1 is prepared according to the following reaction equation
[0060]
[0061] Add 7-hydroxy-3-isopropoxy-naphthocoumarin (160mg, 0.5mmol), diisopropylethylamine (DIPEA, 129mg, 1mmol), 4mL shown in formula 1-1 into the 25mL reaction tube Dichloromethane, Tf at 0°C 2 O (212mg, 1.5mmol) was dropped into the reaction, slowly raised to room temperature and reacted for 1 hour, the solvent was recovered by distillation under reduced pressure, separated and purified by silica gel column chromatography (the eluent was petroleum ether and ethyl acetate volume ratio 50:1~10 :1 mixed solution), obtain formula 7-1 compound. The compound of formula 7-1 is a yellow solid with a melting point of 150.0-150.8°C and a yield of 95%. The structural characterization results are as follows: 1 H NMR (400MHz, CDCl 3 )δ8.35(s,1H),8.20(d,J=8.5Hz,1H),7.98(d,J=9.0Hz,1H),7.92(d,J=8.4Hz,1H),7.72–7.66( m,1H),7.66–7.60(m,1H),6.88(dd,J=9.0,2.5Hz,1H),6.76(d,J=2.5Hz,1H),4...
Embodiment 3
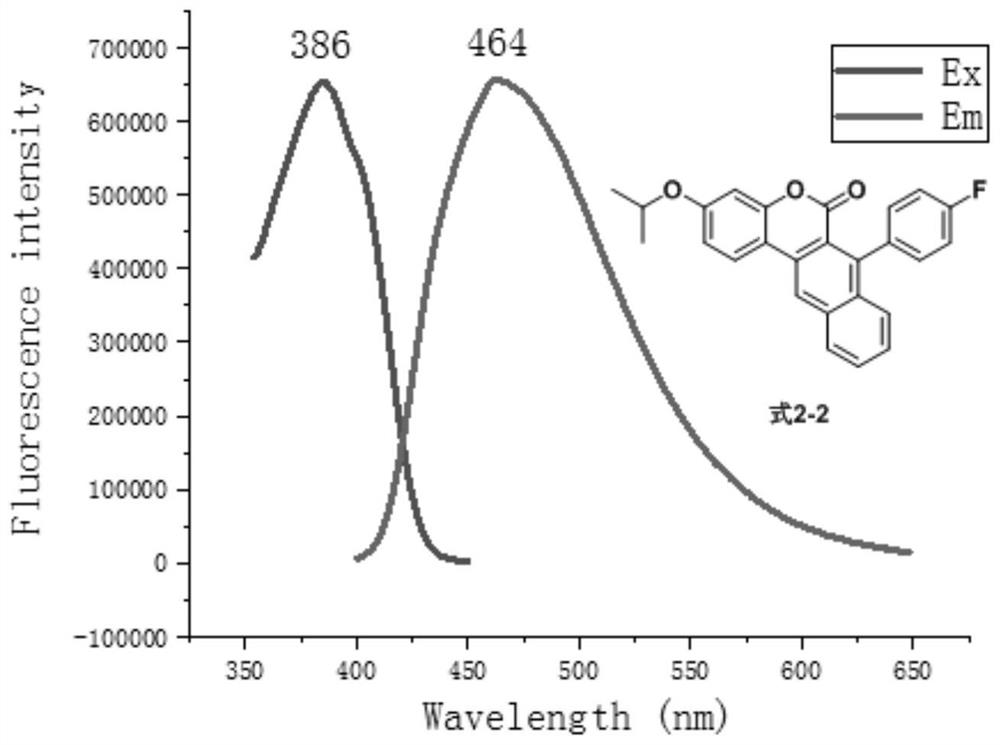
[0070] Prepare formula 3-1 compound according to following reaction equation
[0071]
[0072] Add 7-trifluoromethanesulfonate group-3-isopropoxy-naphthocoumarin (226mg, 0.5mmol) and phenylacetylene (76mg, 0.75mmol) shown in formula 7-1 to the 25mL reaction tube , Pd(PPh 3 ) 2 Cl 2 (17mg, 0.025mmol), CuI (170mg, 0.03mmol), 10mL Et 3 N, react under argon at 90°C for 12 hours, distill under reduced pressure to recover the solvent, separate and purify by silica gel column chromatography (the eluent is a mixture of petroleum ether and ethyl acetate with a volume ratio of 50:1 to 10:1) , to obtain the compound of formula 3-1.
[0073] In the preparation process of the above-mentioned compound of formula 3-1, phenylacetylene is replaced with equimolar 4-fluorophenylacetylene, 4-trifluoromethylphenylacetylene, 4-cyanophenylacetylene respectively, and other steps are the same as those of the compound of formula 3-1. The preparation is the same, and the compounds of formula 3-2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com