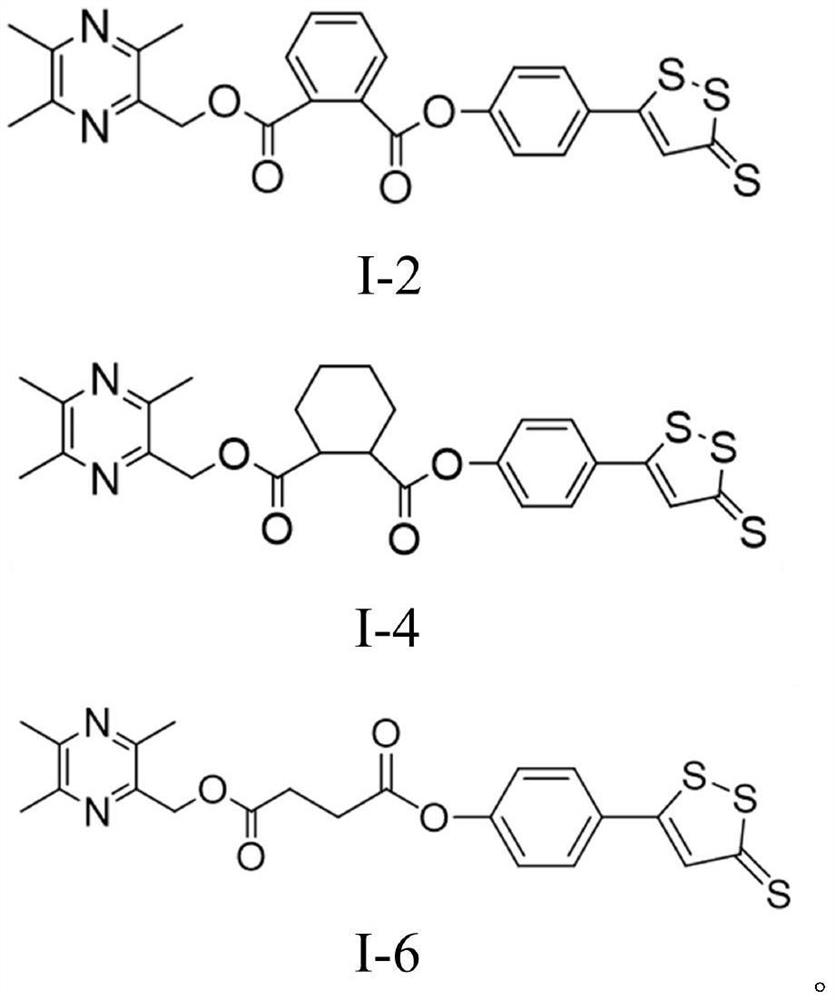
Ligustrazine derivative and preparation method and medical application thereof
A technology of ligustrazine and its derivatives, applied in the field of medicinal chemistry, can solve the problems of weak clinical physiological activity of ligustrazine, limited wide use, and poor water solubility of ligustrazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
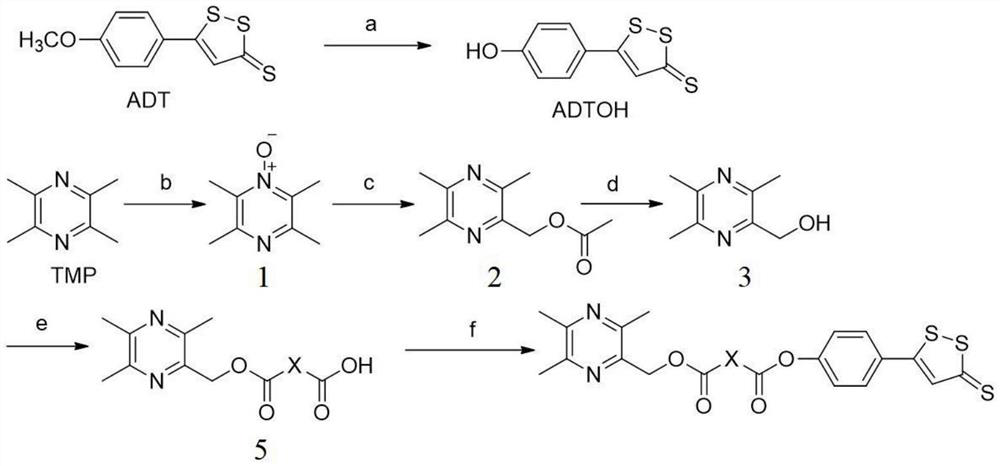
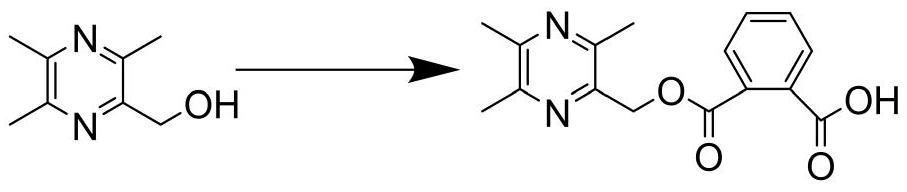
[0046] Embodiment 1: the synthesis of target compound
[0047] (1) Synthesis of 5-p-hydroxyphenyl-3H-1,2-dithiole-3-thione (ADTOH)
[0048]
[0049] Weigh ADT (8g, 33mmol) and pyridine hydrochloride (23g, 200mmol) into a 250ml three-neck flask, heat at 215°C for 0.5h in a constant temperature stirrer, and monitor the completion of the reaction by TLC. After cooling down to 100°C, filter with hot water and take the filter cake. Dissolve the filter cake in 200ml 10% NaOH solution, and stir at room temperature for 4h. The reaction solution was filtered, and the filter cake was taken. Dissolve the filter cake in water, adjust the pH to 2-3 with hydrochloric acid while stirring, and a red precipitate is formed at this time. Continue to filter, wash the filter cake until neutral, and dry to obtain orange-yellow solid ADTOH with a yield of 64%.
[0050] (2) Synthesis of (3,5,6-trimethylpyrazin-2-yl)methanol (3)
[0051]
[0052] Ligustrazine (500mg, 0.027mmol) and 2ml of h...
Embodiment 2
[0071] Example 2: Evaluation of anti-platelet aggregation activity
[0072] Take 2 rabbits, use lidocaine for local anesthesia, surgically separate the common carotid artery, take blood, take 3.8% sodium citrate 1:9 anticoagulation, and centrifuge at 500r / min for 10min to prepare platelet-rich plasma (PRP). Part of it was centrifuged at 3000r / min to prepare platelet-poor plasma (PPP), and the platelet aggregation experiment was carried out by turbidimetric method. Add 240 μL of PRP and 30 μL of different concentrations of test drugs into the measurement tube, and incubate for 5 min. ) (final concentration 1mmol / L) as the inducer, observe and record the maximum aggregation rate within 5min. Using normal saline (NS) as a control, calculate the inhibitory rate (%) or IC of each test compound 50 value. Inhibition rate (%) = [the maximum aggregation rate within 5 minutes of the control group - the maximum aggregation rate within 5 minutes of the test sample group] / [the maximum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com