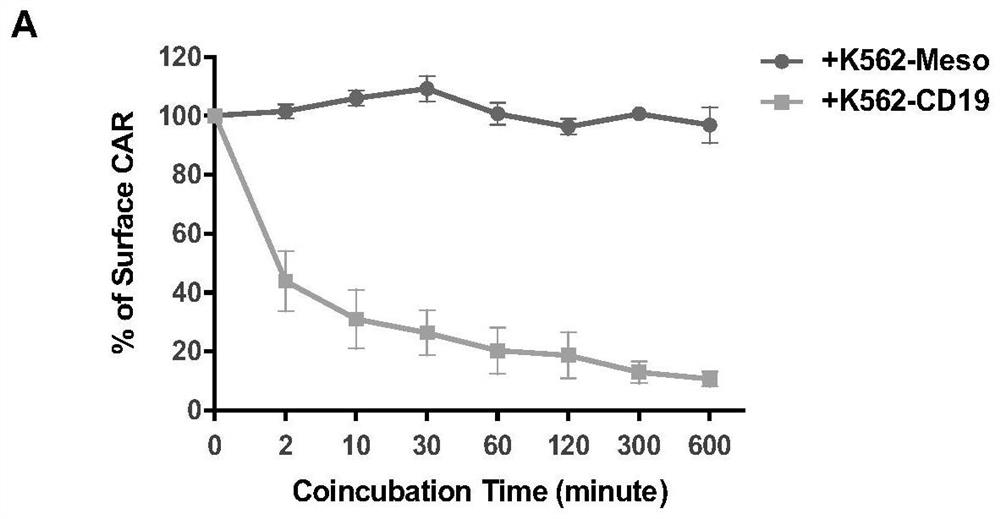
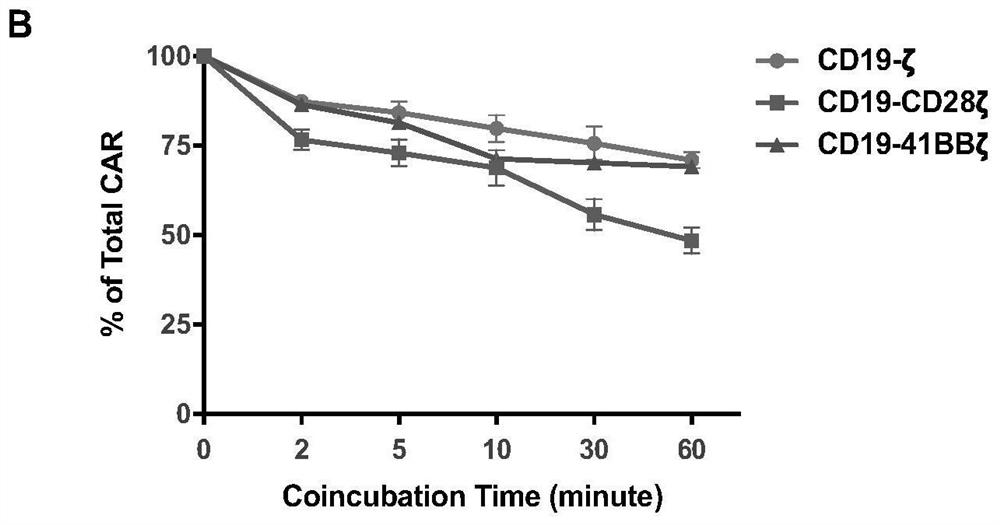
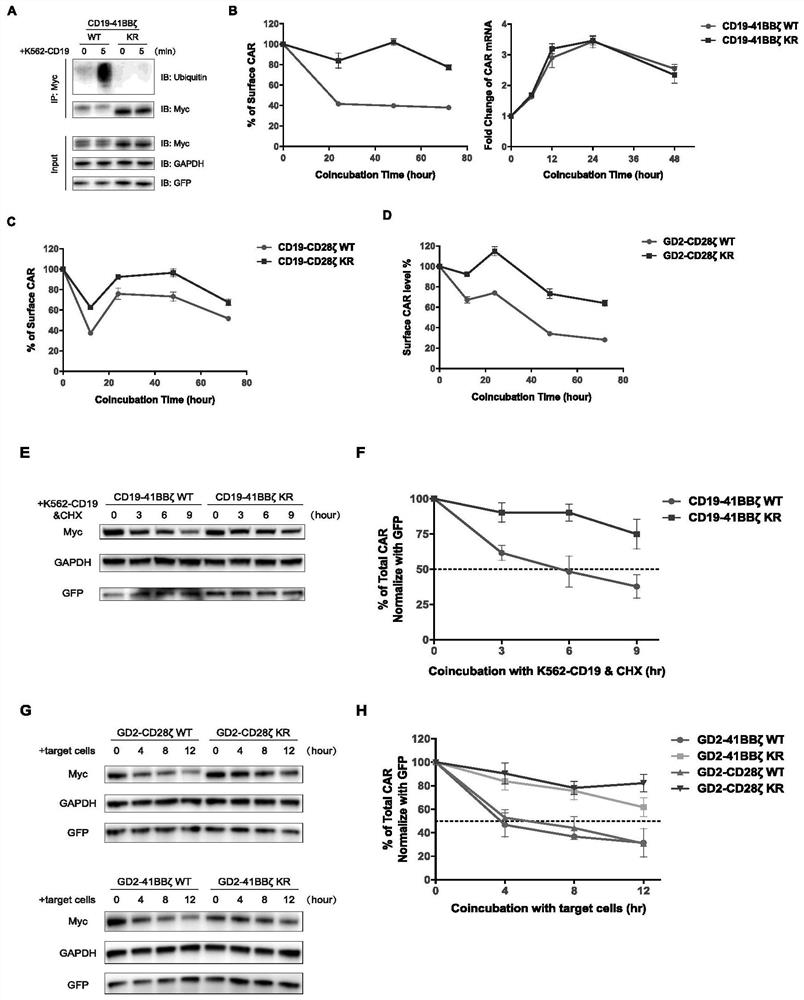
A kind of ubiquitination-deficient chimeric antigen receptor and its use
A chimeric antigen receptor and antigen recognition technology, which can be applied to cancer antigen components, vertebrate antigen components, and medical preparations containing active ingredients, etc. Low transformation efficiency and other problems, to achieve the effect of strong killing activity, enhanced survival and proliferation ability, strong mitochondrial production capacity and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: Vector construction of CAR
[0130] The antigen-specific single chain antibody (scFv) sequences of CD19 and GD2CAR used in the present invention are derived from the clinically used FMC63 and 14g2a sequences, respectively. The extracellular segment structure of CAR is composed of CD8α signal peptide sequence, myc tag sequence, scFv sequence, and CD8α hinge sequence; the transmembrane region sequence is the transmembrane region sequence of CD8α; the intracellular segment structure is composed of human 41BB intracellular segment sequence. Or CD28 intracellular segment sequence in series with human CD3ζ intracellular segment sequence. The above amino acid sequence and the amino acid sequence in which the intracellular lysine was mutated to arginine were all converted into base sequences after codon optimization and synthesized by the company (Qinglan Biotech). The base sequences of all CARs in the present invention are finally cloned into the pHR-hEF1α-IRES-EGF...
Embodiment 2
[0131] Example 2: Human primary T cell culture and lentiviral infection
[0132] Human primary T cells were obtained from healthy informed volunteers. Primary T cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin sulfate (all reagents were purchased from Gibco). To maintain the proliferation of T cells, 100 U / ml of hIL-2 (Sigma-Aldrich) was added to the medium.
[0133] Preparation of lentivirus: 2.2 × 10 5 Individual Lenti-X 293T cells (TaKaRa #632180) were resuspended in DMEM medium (Gibco #11995-065) containing 10% fetal bovine serum and cultured in 6-well cell culture plates (Corning #CLS3516) for 24 hours. Using the lipofection system (Mirus#2300), 500ng of lentiviral packaging plasmids pCMVdR8.92 (Addgene#8455) and pMD2.G (Addgene#12259) were transfected with 500ng of the lentiviral plasmids to be packaged according to liposomes The operating steps of the instructions were added to the Lenti-X 293T ...
Embodiment 3
[0135] Example 3: Flow Cytometry Analysis
[0136] For staining of cell surface markers: Antibodies were diluted in FACS buffer (phosphate buffered saline PBS + 2% fetal bovine serum) and incubated with cells for 25 minutes at 4°C in the dark.
[0137] For staining containing intracellular markers: cells were first fixed with 4% paraformaldehyde (Meilunbio #MA0192) for 15 minutes at room temperature, then the membranes were ruptured using pre-chilled methanol for 50 minutes on ice. Staining was then performed by incubation with antibody dilutions (same as surface staining) for 60 minutes in the dark at room temperature. The Zombie Violet Fixableviability kit (Biolegend #423114) was used to differentiate the dead and live states of the cells.
[0138] Flow cytometry data were acquired by a BD LSRFortessa machine (BD bioscience) and data analysis was performed using FlowJosoftware software (Tree Star). Antibody information for flow cytometry is listed below.
[0139]
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com