Method for improving extracellular secretion level of recombinant protein of escherichia coli
A technology of Escherichia coli and recombinant protein, which is applied in the fields of genetic engineering and fermentation engineering, and can solve problems such as the unclear effect of recombinant protein extracellular secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Construction process of recombinant Escherichia coli integrating different numbers of SD sequences
[0058] (1) Using the plasmid pKD13 as a template, design primers and PCR amplify the homologous fragments containing the Kan resistance gene for replacing the 1SD, 2SD, 3SD, and 4SD sequences. The sequences are respectively as SEQ ID NO.1, SEQ ID NO. 2. As shown in SEQ ID NO.3 and SEQ ID NO.4, the Kan resistance gene contains FRT sites on both sides, and different SD sequence integration frame fragments are obtained.
[0059] (2) Transform the pKD46 plasmid into E.coli BL21(DE3) competent cells to obtain E.coli BL21(DE3) / pKD46 strains, prepare electroporation competent cells, and electroporate the integrated frame fragments prepared in step (1) Transformed into E.coli BL21(DE3) / pKD46 competent medium, the transformation solution was post-cultivated and spread on LB solid medium containing kanamycin (Kan) and ampicillin to obtain the transformant BL21::1SD: :ka...
Embodiment 2
[0061] Example 2 The effect of different distances from gene integration SD sequence to the initial codon ATG of carboxypeptidase DacA on the extracellular production of recombinant protein in E.coli
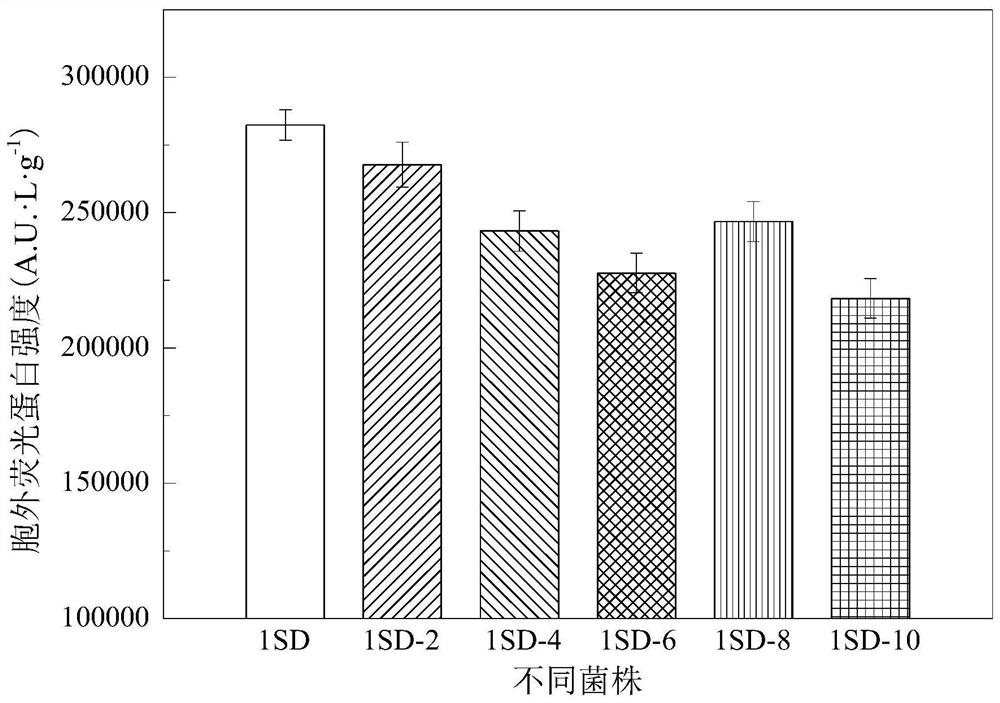
[0062] In this example, the effect of different distances from the gene integration 1SD sequence to the initial codon ATG of carboxypeptidase DacA on the extracellular production of recombinant protein by E. coli was determined and analyzed. In this example, GFP was used as a model protein for verification. Recombinant strains with distances from the initial codon ATG of DacA of 0, 2, 4, 6, 8, and 10 bases were respectively constructed to obtain recombinant strains BL21(+1SD), BL21(+1SD)-2, BL21(+1SD )-4, BL21(+1SD)-6, BL21(+1SD)-8, BL21(+1SD)-10, and transform the recombinant plasmid pET28a-gfp respectively. Such as figure 2 As shown, the extracellular secretion level of BL21(+1SD)-gfp recombinant GFP is the highest when the distance is 0 bases, and its extracellular GFP flu...
Embodiment 3
[0063] Example 3 Extracellular Production of Recombinant Green Fluorescent Protein by Recombinant Escherichia coli
[0064] In this example, the effect of gene integration on the extracellular production of GFP in E. coli was determined and analyzed.
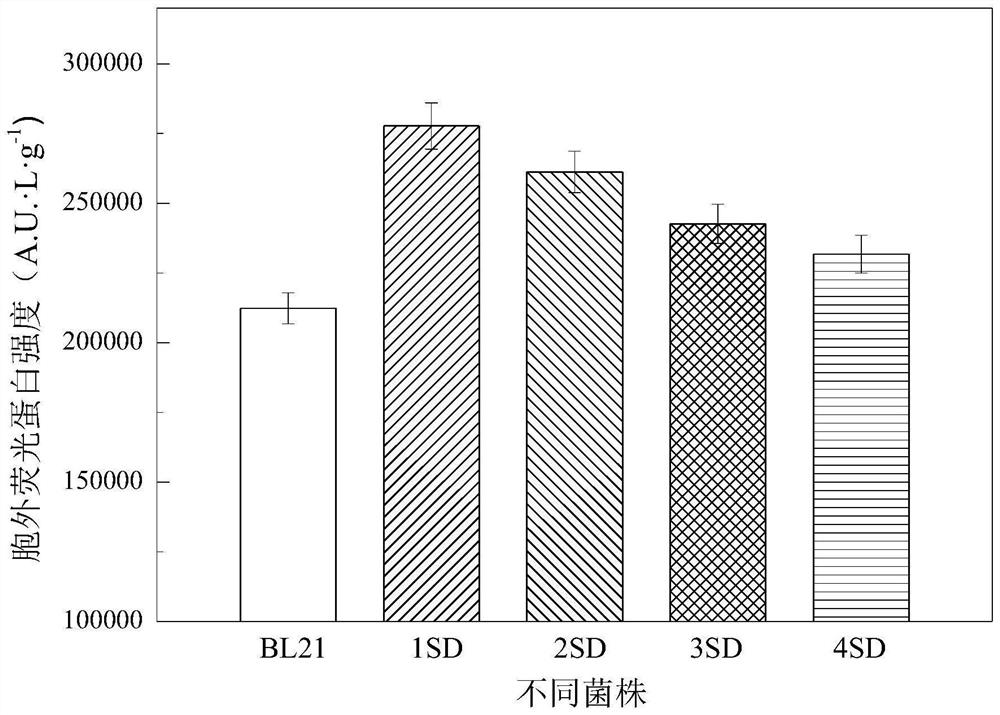
[0065]The recombinant plasmid pET28a-gfp was respectively transformed into the starting strain BL21, the BL21(+1SD) and BL21(+2SD) strains constructed in Example 1, and the recombinant mutants BL21-gfp, BL21(+1SD)-gfp and BL21( +2SD)-gfp. The recombinant mutants BL21-gfp, BL21(+1SD)-gfp and BL21(+2SD)-gfp were cultured in LB medium at 37°C for 8h, and then inoculated into TB medium at an inoculation amount of 1% (v / v) medium, cultured at 37°C to OD 600 =0.8, the final concentration of adding is 1mmol·L -1 IPTG, induced expression at 25°C.
[0066] Measure the amount of GFP fluorescence. Such as image 3 As shown, the extracellular GFP fluorescence values of BL21(+1SD)-gfp and BL21(+2SD)-gfp mutant strains were 2.8×10 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com