Method for reducing NAD analogue by using methanol
An analogue, methanol technology, applied in the direction of fermentation, etc., can solve the problem of transforming methanol dehydrogenase that has not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Using methanol as a reducing agent, methanol dehydrogenase catalyzes the reduction of NAD analogs
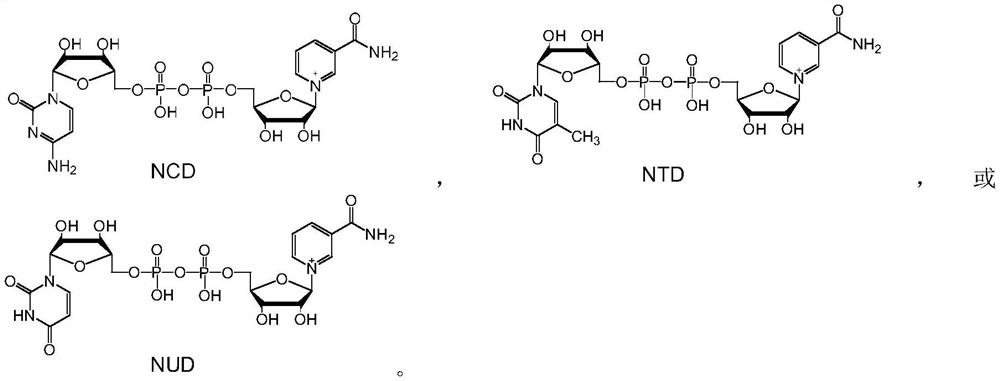
[0034] NAD and its analog NCD, NTD or NUD, and methanol dehydrogenase BsMDH, BsMDH-V237T, BsMDH-V237T / N240E, BsMDH-V237T / N240E / K241A, BsMDH-Y171R / V237T / N240E / K241A, BsMDH-Y171R / I196V / V237T / N240E / K241A, carry out NAD analogue-methanol dehydrogenase combination one by one, and react as follows: dissolve 1mM NAD or analogue, 8mM methanol and 80μg methanol dehydrogenase in 1mL concentration 50mM, pH 7.5 HEPES buffer, mix well, react at 30°C for 20min, take 20μL for analysis.
[0035] According to the analysis method of Comparative Example 1, it was found that all the samples used had characteristic absorption peaks at 340 nm, but the intensity of absorption peaks obtained by different combinations was significantly different, indicating that methanol dehydrogenase can catalyze the reduction of NAD analogues by methanol. Due to the molar extinction coefficient ε o...
Embodiment 2
[0040] Example 2: Preparation of reduced NAD analogs
[0041] The reaction system in Example 1 is scaled up and can be used to prepare reduced NAD analogs. Take NUDH as an example to illustrate the preparation process. Dissolve 20 mM NUD, 20 mM methanol and 5 mg methanol dehydrogenase BsMDH-V237T in 10 mL of sodium phosphate buffer solution with a concentration of 50 mM and a pH of 5.7, mix well, and react at 30°C for 80 min. Freeze-dry directly after the reaction, concentrate to a total volume of about 4 mL, separate with a formic acid-type anion-exchange resin column, track and collect the product at an ultraviolet wavelength of 340 nm, and freeze-dry to obtain 5.6 mg of white powder with a yield of about 44%.
[0042] The above-mentioned white powder sample was subjected to high-resolution mass spectrometry analysis to measure the precise molecular weight (M+H) + It is 643.1026, and the theoretical molecular weight of NUDH (C 20 h 29 N 4 o 16 P 2 + , 643.1054) consi...
Embodiment 3
[0044] Example 3: Using methanol as a reducing agent, methanol dehydrogenase catalyzes the reduction of NAD analogs
[0045] Dissolve 0.1 mM NUD, 0.8 mM methanol and 80 μg methanol dehydrogenase BsMDH-V237T / N240E in 1 mL of 50 mM, pH 8.0 PIPES buffer, mix well, react at 40°C for 3 min, and take 20 μL for analysis.
[0046] Analysis by the method of Comparative Example 1 found that the sample had a characteristic absorption peak at 340nm. The concentration of generated NBrCDH reached 60 μM, that is, the yield reached 60%.
[0047] The results of Example 1 and Example 3 show that in the reaction of methanol dehydrogenase catalyzed reduction of NAD analogues, methanol can be used as a reducing agent to reduce NAD analogues.
[0048] According to the method of Example 3, NAD analogs were produced with the same amount of NUD, sodium phosphite and phosphite dehydrogenase rsPDH-I151R, and the concentration of NUDH generated reached 43 μM, that is, the yield reached 40%. It shows th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com