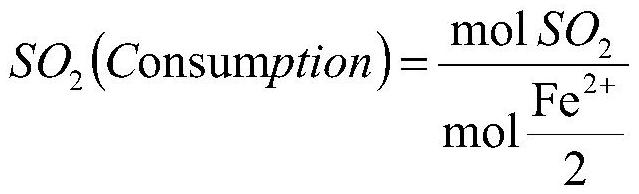Method for removing iron in waste nickel-metal hydride battery
A nickel-metal hydride battery, waste technology, applied in battery recycling, chemical instruments and methods, nickel compounds, etc., can solve problems such as no new treatment plan reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
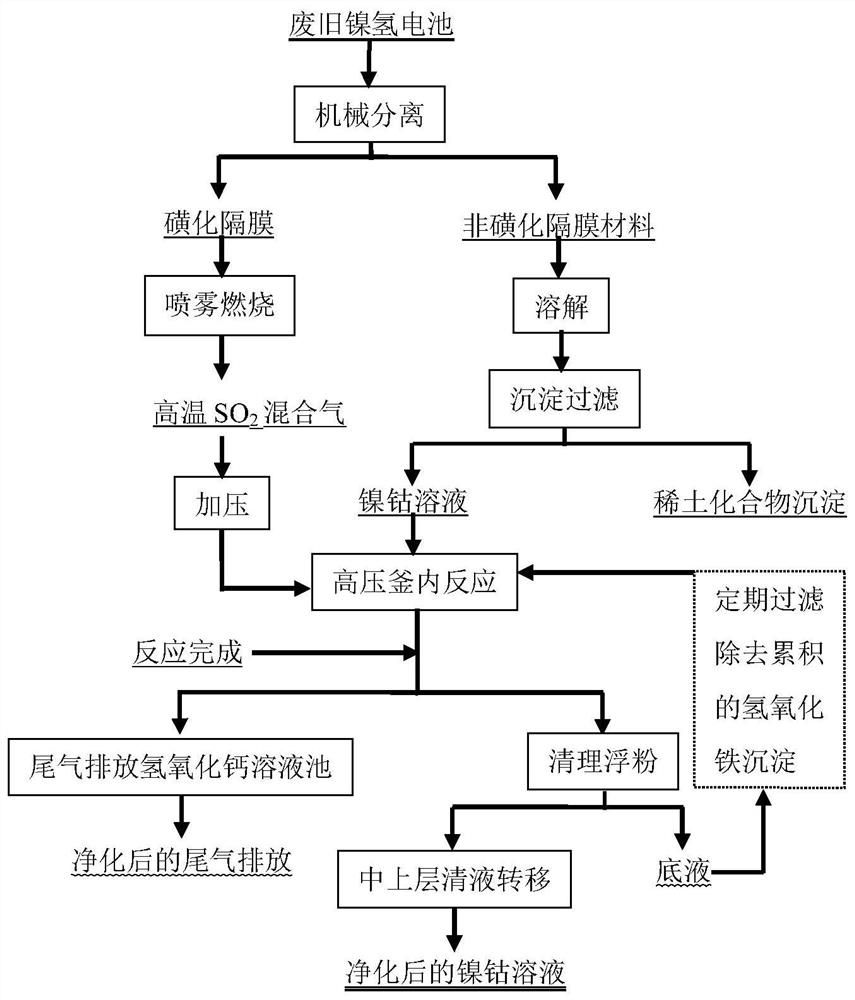
[0059] A method for removing iron in waste nickel-metal hydride batteries, comprising the steps of:
[0060] (1) Using a mechanical cyclone to separate waste nickel-metal hydride batteries into sulfonated diaphragm and non-sulfonated diaphragm materials with a sulfur mass fraction of 0.5%;
[0061] (2) Dissolve the non-sulfonated diaphragm material in a mixed solution of sulfuric acid and hydrogen peroxide, wherein the molar ratio of sulfuric acid and hydrogen peroxide is 10:0.5, then use sodium hydroxide to precipitate rare earth, and separate the rare earth compound by filtration to obtain a nickel-cobalt solution , the pH value is 5.0;
[0062] (3) Finely crush the sulfonated diaphragm to a particle size of 5mm, spray it into the combustion furnace through a spray device, and carry out combustion under an oxygen-enriched air atmosphere; the mixed gas generated by combustion is pressurized by a compressor, and the pressurized pressure is 0.15MPa; The pressurized mixed gas i...
Embodiment 2
[0088] A method for removing iron in waste nickel-metal hydride batteries, comprising the steps of:
[0089] (1) Using a mechanical cyclone to separate waste nickel-metal hydride batteries into sulfonated diaphragm and non-sulfonated diaphragm materials with a sulfur mass fraction of 0.2%;
[0090] (2) Dissolve the non-sulfonated diaphragm material in a mixed solution of sulfuric acid and hydrogen peroxide, wherein the molar ratio of sulfuric acid and hydrogen peroxide is 10:0.3, then use sodium hydroxide to precipitate rare earth, and separate the rare earth compound by filtration to obtain a nickel-cobalt solution , the pH value is 4.5;
[0091] (3) Finely crush the sulfonated diaphragm to a particle size of 1mm, spray it into the combustion furnace through a spray device, and carry out combustion under an oxygen-enriched air atmosphere; the mixed gas generated by the combustion is pressurized by a compressor, and the pressurized pressure is 0.1MPa; The pressurized mixed ga...
Embodiment 3
[0096] A method for removing iron in waste nickel-metal hydride batteries, comprising the steps of:
[0097] (1) Using a mechanical cyclone to separate waste nickel-metal hydride batteries into sulfonated diaphragm and non-sulfonated diaphragm materials with a sulfur mass fraction of 0.3%;
[0098] (2) Dissolve the non-sulfonated diaphragm material in a mixed solution of sulfuric acid and hydrogen peroxide, wherein the molar ratio of sulfuric acid and hydrogen peroxide is 10:0.8, then use sodium hydroxide to precipitate rare earth, and obtain a nickel-cobalt solution after filtration, with a pH value of 4.8 ;
[0099] (3) Finely crush the sulfonated diaphragm to a particle size of 8mm, spray it into the combustion furnace through a spray device, and carry out combustion under an oxygen-enriched air atmosphere; the mixed gas generated by combustion is pressurized by a compressor, and the pressurized pressure is 0.12MPa; The pressurized mixed gas is passed into the autoclave to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com