N-acetyl-5-hydroxytryptamine O-methyltransferase (ASMT12) in Morus notabili and application thereof
A technology of serotonin oxygen methyl and transferase, applied in the direction of transferase, application, recombinant DNA technology, etc., can solve the problems of not being able to obtain a large amount of extraction and low content, and achieve the effect of high enzyme activity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, N-acetyl-5-hydroxytryptamine oxygen methyltransferase gene ASMT12 clone
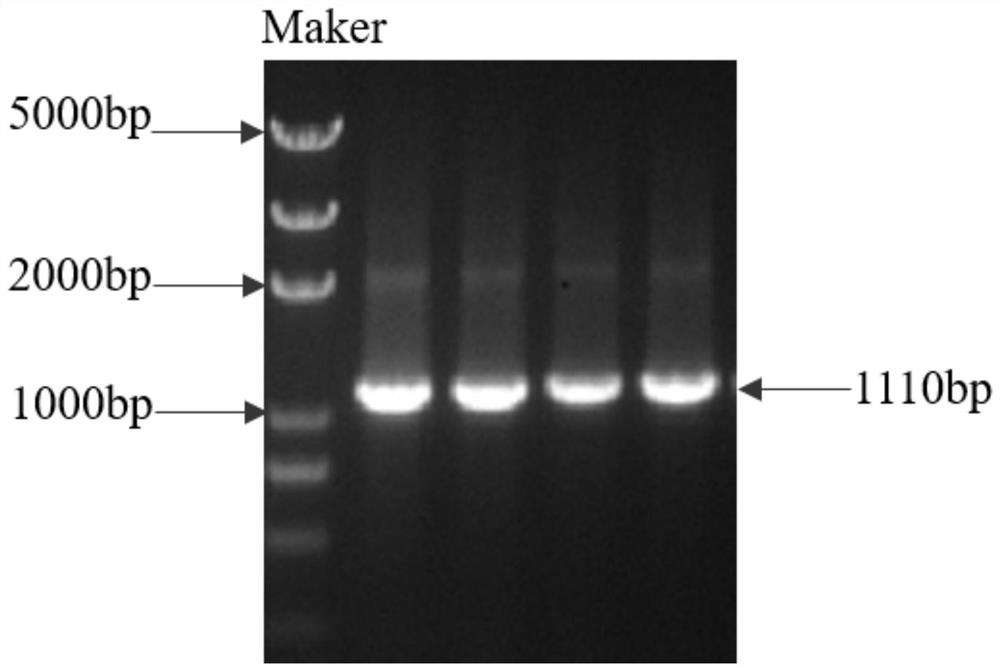
[0026] According to the Morus.ASMT12 N-acetyl-5-hydroxytryptamine oxygen-methyltransferase gene (Morus015040) reported on the Morus.ASMT12 database, the primers for cloning the N-acetyl-5-hydroxytryptamine oxygen-methyltransferase gene ASMT12 were designed, and the upstream primers of ASMT12 were :5'-cg ggatcc atgctgtccttcataagttccatgt-3' (SEQ ID NO.1); the downstream primer of ASMT12 is: 5'-acgc gtcgac tcatttaagcaattcataaac-3' (SEQ ID NO. 2). The cDNA of Chuan Sang was used as a template, and the sequences shown in SEQ ID NO.1 and SEQ ID NO.2 were used as primers for PCR amplification. The amplified products were subjected to agarose gel electrophoresis, and the results were as follows: figure 1shown. The full-length ASMT12 gene was amplified, the recovered product was ligated with the pMD19-T vector, and transformed into E.coli.Trans1-T1 competent cells, and the obtained positiv...
Embodiment 2
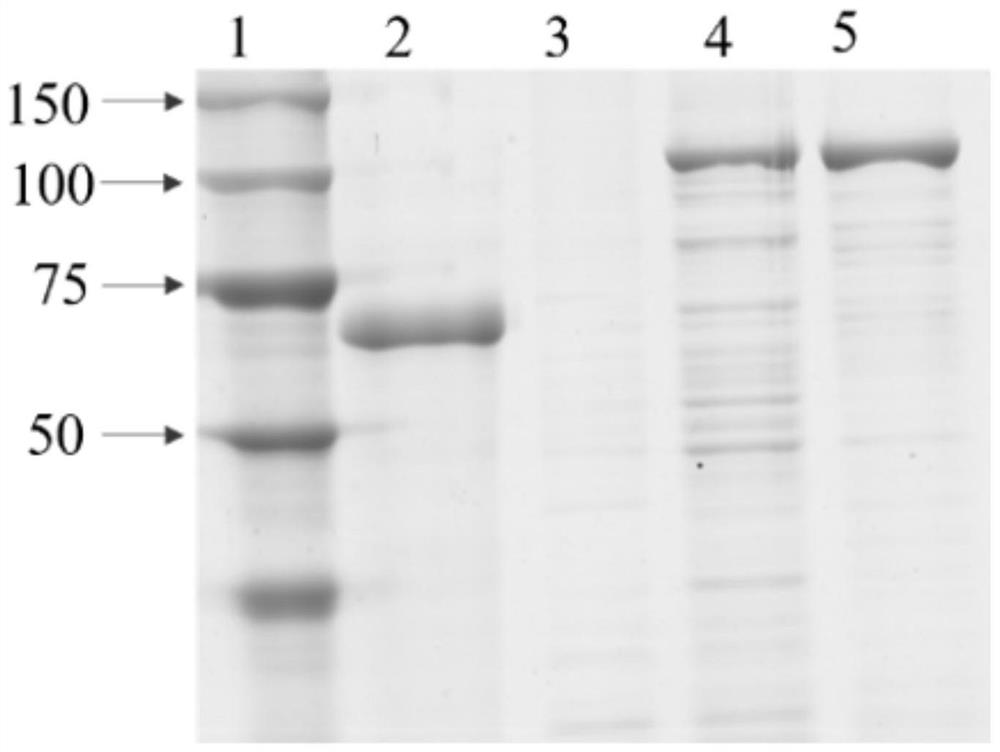
[0027] Example 2. Construction and prokaryotic expression of the ASMT12 recombinant vector of N-acetyl-5-hydroxytryptamine oxygen methyltransferase gene ASMT12 in Chuanmulus. The target fragment of the ASMT12 gene cloned in Example 1 and the Pcold-tf empty plasmid were double-digested with BamHI and SalI, respectively. , recovering the product with T 4 Connect it with DNA ligase to obtain the recombinant vector Pcold-tf-ASMT12, transform the obtained recombinant vector Pcold-tf-ASMT12 into Escherichia coli DH5ɑ competent cells, and send the correct identified Pcold-tf-ASMT12 to Huada Gene Company Sequencing, the sequencing results were consistent with the sequence obtained by the first sequencing so that the correct sequence was obtained.
[0028] Extract the Pcold-tf-ASMT12 plasmid, transfer it into the expression strain B21(DE3), add 450μl LB medium containing Amp resistance to a 1.5ml centrifuge tube, and expand the culture into the test tube at a ratio of 1:100, 28°C, 220r...
Embodiment 3
[0030] Example 3, concentration and activity detection of recombinant N-acetyl-5-hydroxytryptamine oxygen methyltransferase ASMT12
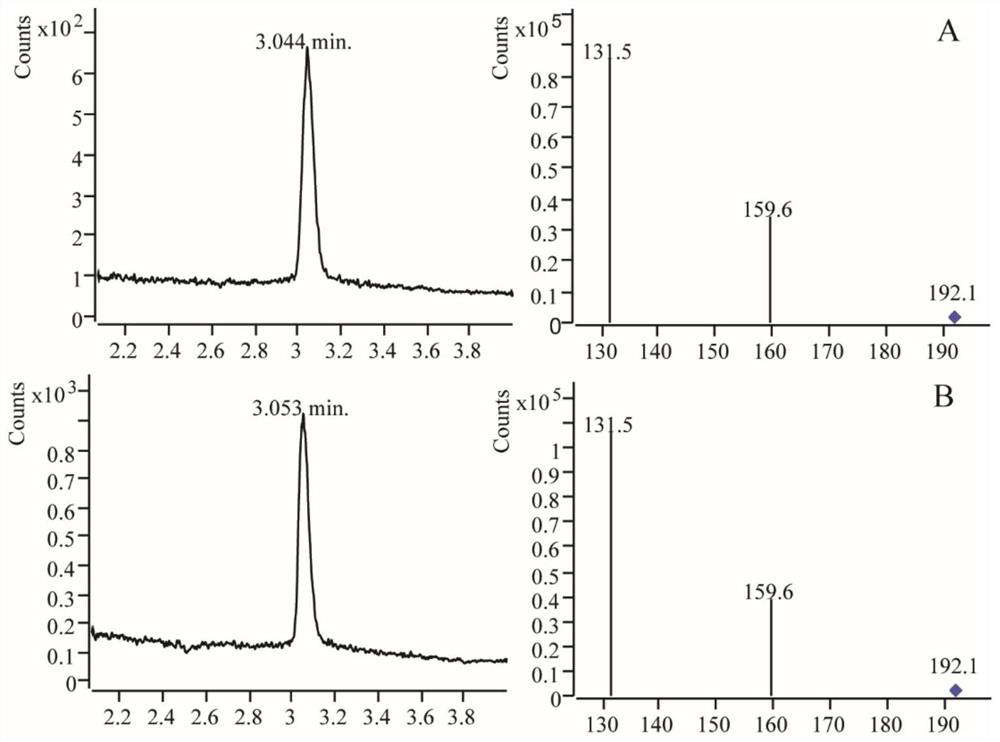
[0031] Take the supernatant induced by the strain containing Pcold-tf-ASMT12 plasmid for 8 hours and purify it through a nickel column, and then use UPLC to measure the amount of the product of the enzymatic reaction to indicate the enzyme activity. The unit of enzyme activity is defined as the substance that generates 1 nmol per minute The amount is a specific activity, that is, after shaking 1U of the induced bacterial solution for 8 hours, 5ml of the supernatant is purified, and then eluted with 10ml of imidazole at a concentration of 100mM. After the liquid is eluted, the N-acetyl- The concentration of 5-hydroxytryptamine oxygen methyltransferase is: the concentration of N-acetyl-5-hydroxytryptamine oxygen methyltransferase ASMT12 is 0.064mg / ml.
[0032] Using 5-hydroxytryptamine at a concentration of 0.05 μM as a substrate, reacted with N-ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com