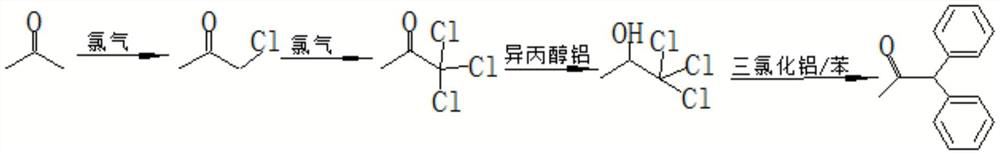
Continuous production process of metadiphenylacetone
A technology for producing diphenylacetone and its production process, which is applied in the field of continuous production process of diphenylacetone, can solve the problems of complex process flow, excessive waste water and waste gas, and high requirements for production equipment, and achieve high yield and purity, Effect of shortening operation time and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
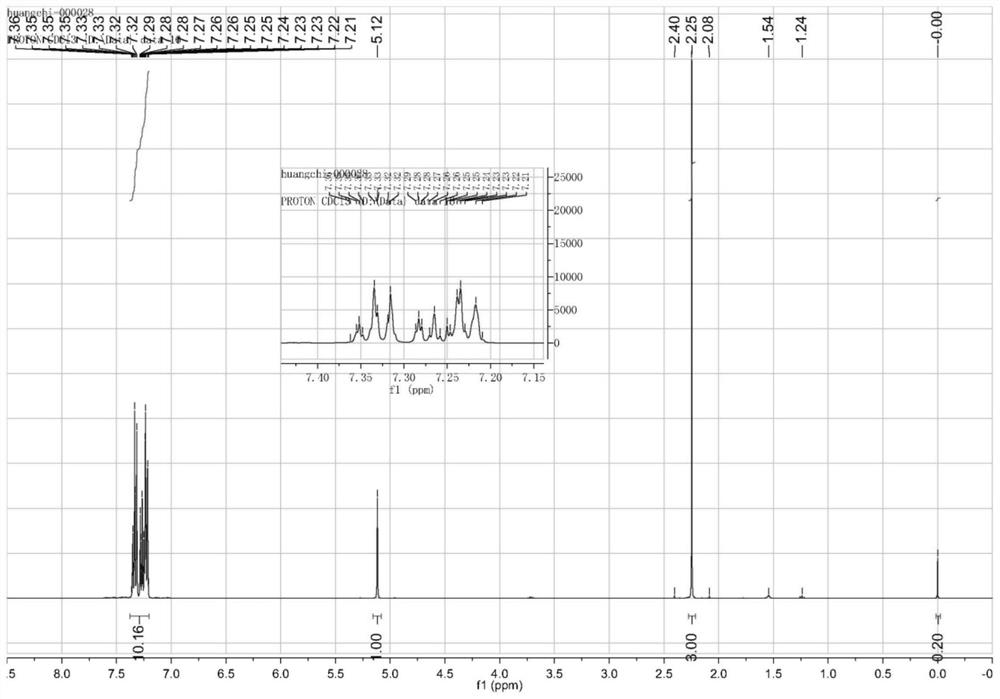
[0047] The continuous production process of the partial diphenylacetone described in the present embodiment 1 is made up of the following steps:
[0048] (1) Under a nitrogen protective atmosphere and an anhydrous dry environment, place 1.7Kg of prefabricated Grignard reagent as an initiator in a Grignard reagent kettle, then add 15.8Kg of anhydrous tetrahydrofuran and 3.5Kg of magnesium chips, heat to slight reflux, drop Add anhydrous tetrahydrofuran solution of chlorobenzene (chlorobenzene 15.2Kg, THF 22.5Kg), keep the reaction system under slight reflux, and prepare the Grignard reagent; after the dropwise addition, continue to react for 4.5 hours, then lower the temperature to room temperature and let stand; The prepared Grignard reagent is transported to the Grignard reactor through pipelines;
[0049] (2) Add 6.4Kg of lactic acid ester to the Grignard reaction kettle, reflux at 105°C for 2 hours, cool down to 10°C, transfer the reaction solution generated by the reaction...
Embodiment 2
[0061] The continuous production process of the partial diphenylacetone described in the present embodiment 2 is made up of the following steps:
[0062] (1) Under a nitrogen protective atmosphere and an anhydrous dry environment, place 1.9Kg of prefabricated Grignard reagent as an initiator in a Grignard reagent kettle, then add 17.2Kg of anhydrous tetrahydrofuran and 3.8Kg of magnesium chips, heat to slight reflux, drop Add anhydrous tetrahydrofuran solution of chlorobenzene (chlorobenzene 17.0Kg, THF 24.4Kg), keep the reaction system under slight reflux, and prepare the Grignard reagent; after the dropwise addition, continue to react for 6 hours, then lower the temperature to room temperature and let stand; The prepared Grignard reagent is transported to the Grignard reactor through pipelines;
[0063] (2) Add 7.0Kg of lactic acid ester to the Grignard reaction kettle, reflux at 100°C for 2.5 hours, cool down to 5°C, transfer the reaction solution generated by the reaction ...
Embodiment 3
[0075] The continuous production process of the partial diphenylacetone described in the present embodiment 3 is made up of the following steps:
[0076] (1) Under a nitrogen protective atmosphere and an anhydrous dry environment, place 1.5Kg of prefabricated Grignard reagent as an initiator in a Grignard reagent kettle, then add 13.5Kg of anhydrous tetrahydrofuran and 3.0Kg of magnesium chips, heat to slight reflux, drop Add anhydrous tetrahydrofuran solution of chlorobenzene (chlorobenzene 13.5Kg, THF 19.2Kg), keep the reaction system under slight reflux, and prepare the Grignard reagent; after the dropwise addition, continue to react for 3 hours, then lower the temperature to room temperature and let stand; The prepared Grignard reagent is transported to the Grignard reactor through pipelines;
[0077] (2) Add 5.5Kg of lactic acid ester to the Grignard reaction kettle, reflux at 95°C for 3.0 hours, cool down to 0°C, transfer the reaction solution generated by the reaction t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com