Method for synthesizing 3, 6-dibromo-9-bromophenyl-9H-carbazole
A technology of phenylcarbazole and synthesis method, which is applied in the chemical field, can solve the problems of increasing the difficulty of purification, increasing the reaction cost, and dark color of the reaction solution, so as to avoid the coupling process and metal residues, reduce the discharge of three wastes, and the reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
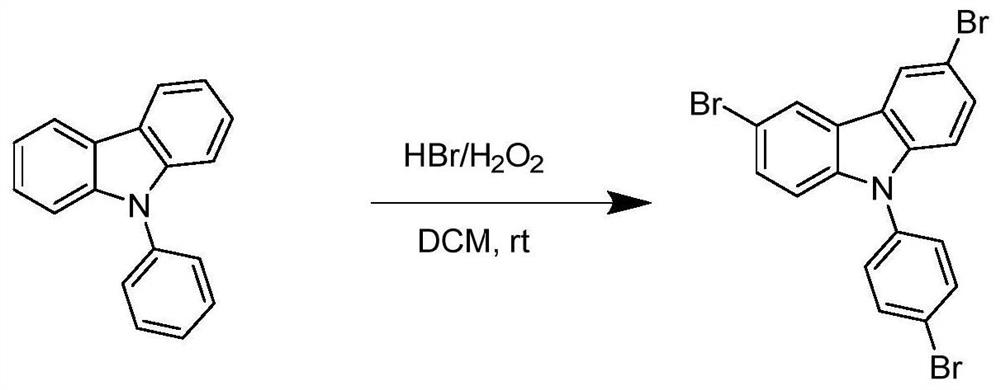
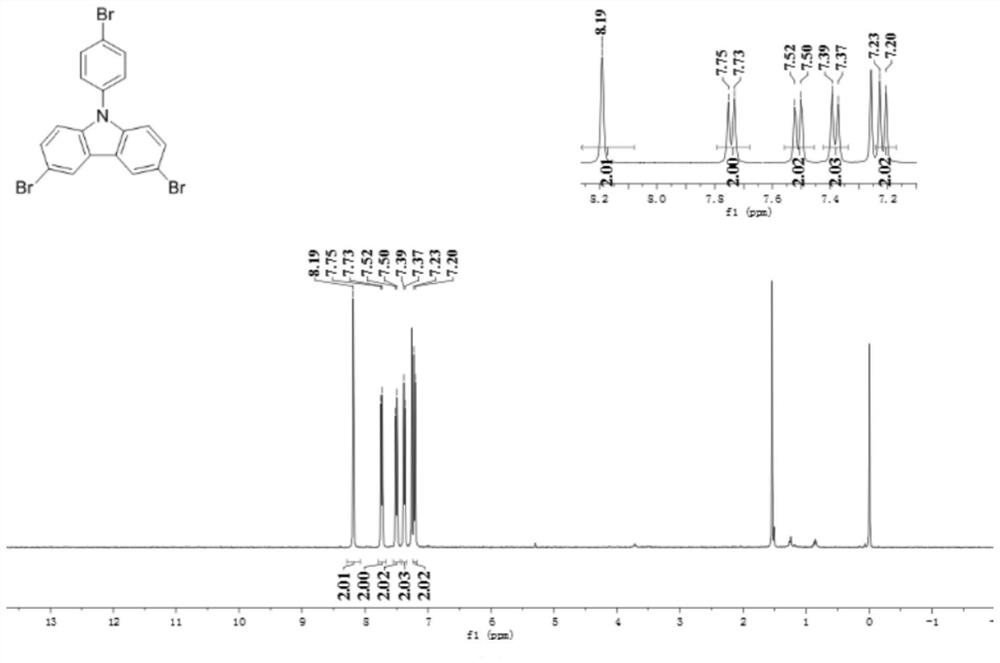
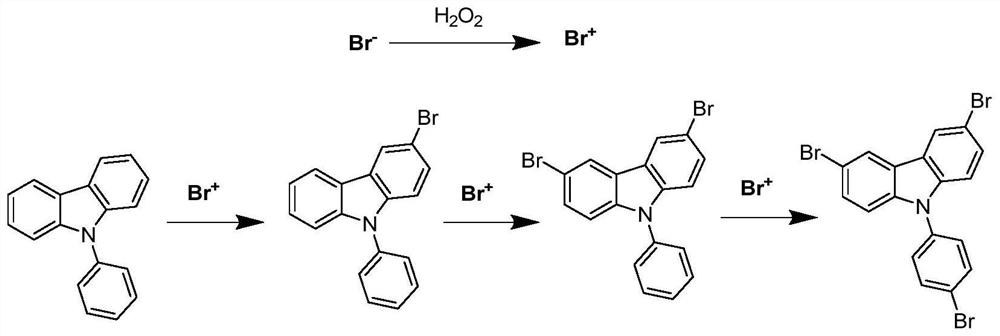
[0045]At room temperature, add 243.3g N-phenylcarbazole (99%, 1mol), 450mL DCM, 667.5g HBr (40%, 3.3mol), rotating speed 550rpm, 374.0g hydrogen peroxide (30%, 3.3 mol) was added dropwise through a constant pressure titration funnel, the rate of addition was 2mL / min, the drop was completed, and the reaction was carried out for 4 hours. Thin-layer chromatography (TLC) was used to monitor whether the reaction raw materials had reacted completely. The crude product was crystallized from ethyl acetate to obtain 406.26 g of tribromo-NPC with a content of 99.5% and a yield of 85.2%. The product was examined by NMR as figure 2 As shown in the figure: 1 H-NMR (400MHz, CDCl 3 ): δ8.19(s, 2H), 7.74(d, J=8.0Hz, 2H), 7.51(d, J=8.0Hz, 2H), 7.38(d, J=8.0Hz, 2H), 7.22(d , J=8.0Hz, 2H), mp=217-218°C. figure 1 It is the reaction equation of the synthesis reaction of this embodiment.
Embodiment 2
[0047] At room temperature, add 243.43g N-phenylcarbazole (99%, 1mol), 450mL DMF, 349.84g NaBr (99.9%, 3.4mol), rotating speed 550rpm, 385.33g hydrogen peroxide (30%, 3.4 mol) was added dropwise through a constant pressure titration funnel at a rate of 1mL / min. After the drop was completed, the reaction was carried out for 5 hours and monitored by thin-layer chromatography (TLC). Crystallization with ethyl acetate gave 344.28 g of tribromo-NPC with a content of 99.3% and a yield of 72.2%.
Embodiment 3
[0049] At room temperature, add 121.63g N-phenylcarbazole (99%, 0.5mol), 250mL DMF, 333.75g HBr (40%, 1.65mol) in 1L reaction flask, rotating speed 550rpm, 187.08g hydrogen peroxide (30%, 1.65mol) was added dropwise through a constant pressure titration funnel at a rate of 2mL / min. After the drop was completed, the reaction was carried out for 5 hours and monitored by thin-layer chromatography (TLC). After the reaction was completed, water and ethyl acetate were added for extraction, and the organic layer was precipitated to obtain a crude Crystallized with ethyl acetate to obtain 149.54 g of tribromo-NPC with a content of 99.6% and a yield of 62.3%. Because the present embodiment adopts polar solvent DMF, DMF has certain complexation with the positive bromide ion that in situ generates, has reduced the electrophilic ability of positive bromide ion, causes reaction yield low (solvent effect); Simultaneously bromine source Amount reduces to some extent compared with embodiment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com