Cyano-modified pyridino imidazole derivative as well as preparation method and application thereof
A technology for pyridoimidazoles and derivatives, which is applied in the fields of organic chemistry, chemical instruments and methods, organic chemistry, etc., can solve the problems of low fluorescence quantum yield and poor solubility, and achieve high fluorescence quantum yield, Good solubility and the effect of suppressing exciton annihilation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
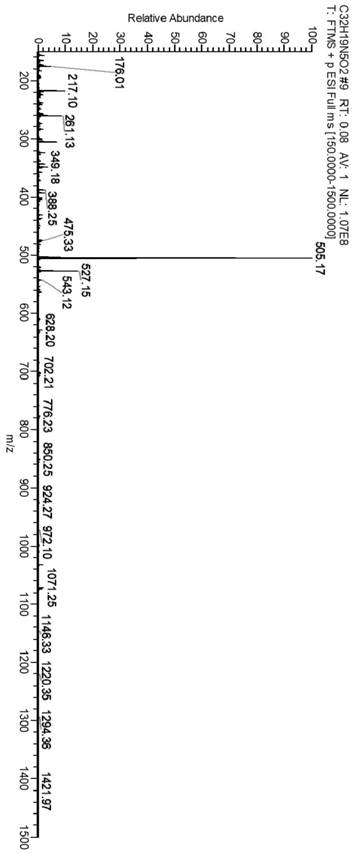
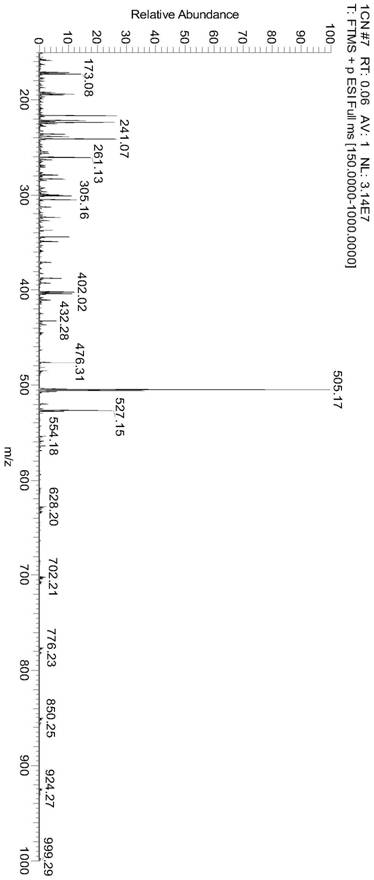
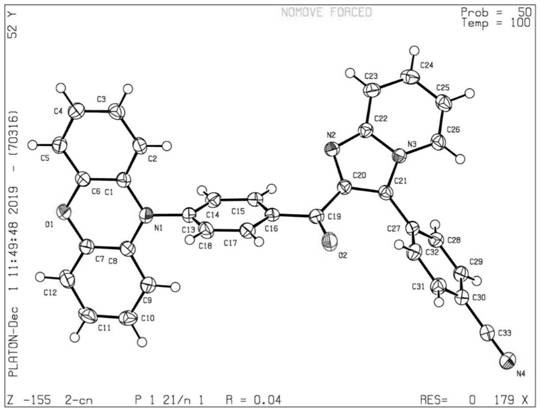
Image
Examples
Embodiment 1
[0071] A cyano-modified pyridoimidazole derivative, named Ben-CN, has the following molecular structure:
[0072]
[0073] The preparation method of the above-mentioned Ben-CN comprises the following steps:
[0074] Weigh 150 mg of (E)-4-(3-(4-bromophenylhydrazine))benzonitrile, 80 mg of 2-aminopyridine, 60 mg of iodine, and 3 mL of dichloroethane in a 10 mL sealed tube, and perform a Michael ring at 120°C. Combined reaction, after processing, (4-(2-(4-bromobenzene) imidazo[1,a])pyridin-3-yl)benzonitrile is obtained; DCE is dichloroethane;
[0075] Its reaction equation is:
[0076]
[0077] Weigh 180 mg of (4-(2-(4-bromobenzene) imidazo[1,a]) pyridin-3-yl) benzonitrile, 115 mg of 10-hydrogen-phenoxazine, and 80 mg of potassium tert-butoxide prepared above , 4 mg of tri-tert-butylphosphine, 5.5 mg of palladium acetate and 5 mL of toluene in a sealed tube, stirred to remove the air in the device and filled with nitrogen protection, heated and stirred under reflux at 130...
Embodiment 2
[0081] A cyano-modified pyridoimidazole derivative, named Bd-CN, has the following molecular structure:
[0082]
[0083] The preparation method of the above-mentioned Bd-CN is the same as in Example 1, the difference is that (E)-4-(3-(4-bromophenylhydrazine))benzonitrile is replaced by (E)-4-(3-(4- Bromophenylhydrazine)) nitrile, 2-aminopyridine was replaced by 2-amino-4-cyano-pyridine to obtain (4-(2-(4-bromophenyl)imidazo[1,a])pyridin-3-yl ) pyridinenitrile;
[0084] Its reaction equation is as follows:
[0085]
Embodiment 3
[0087] A cyano-modified pyridoimidazole derivative with the following molecular structure:
[0088]
[0089] The preparation method of the above-mentioned cyano-modified pyridoimidazole derivatives is the same as that of Example 1, the difference is that (4-(2-(4-bromobenzene)imidazo[1,a])pyridin-3-yl)benzyl The nitrile was replaced by (4-(2-(4-bromophenyl)imidazo[l,a])pyridin-3-yl)benzyl-pyridinenitrile.
[0090] Its reaction equation is as follows:
[0091]
[0092]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com