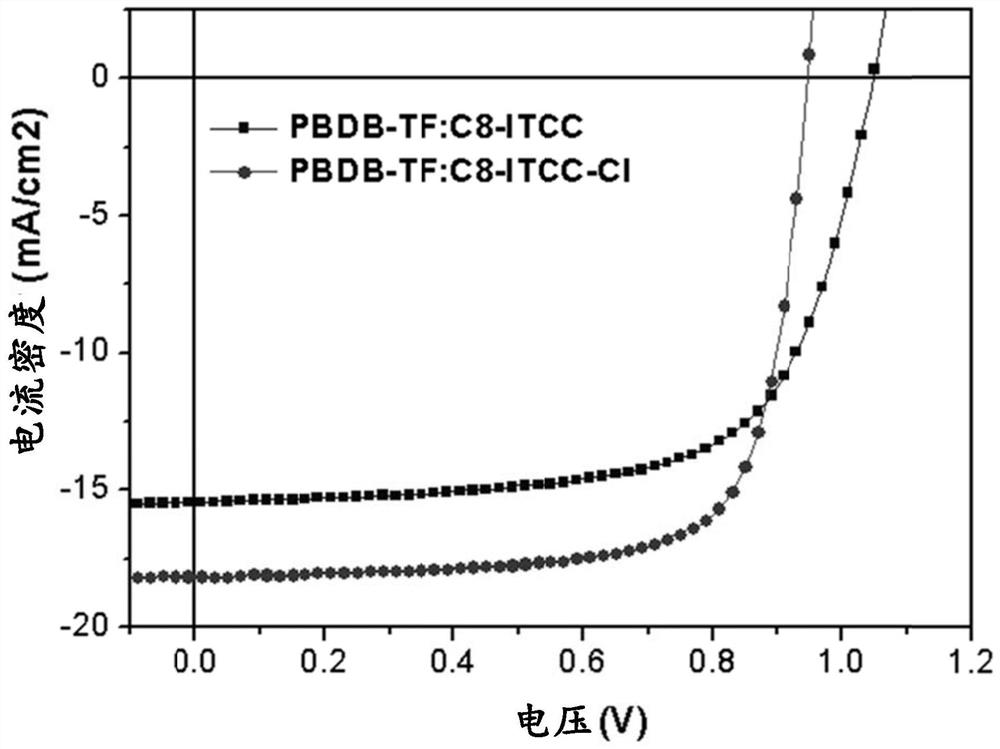
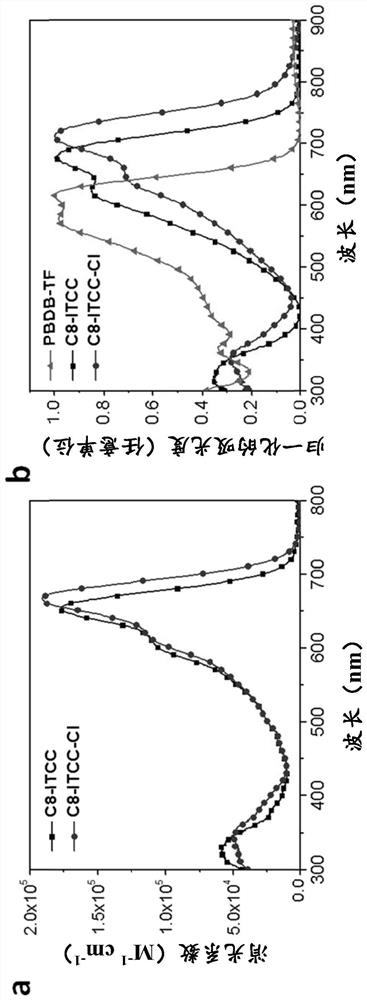
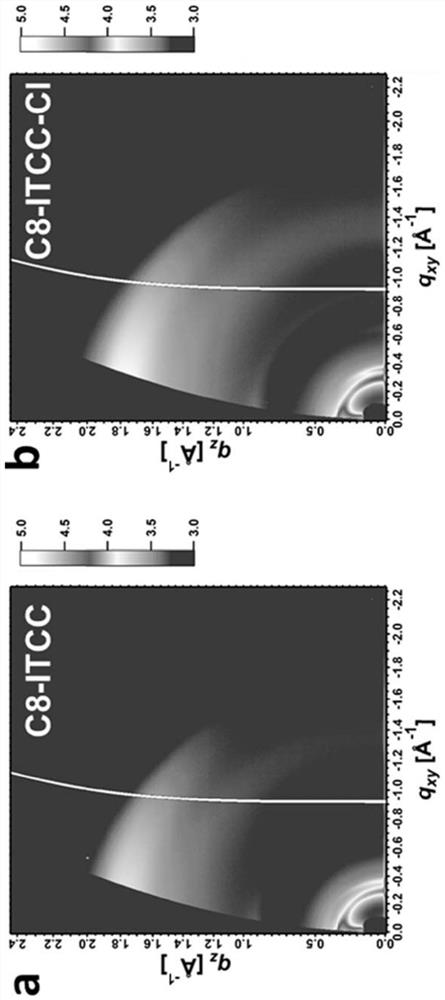
Thiophene end groups of non-fullerene acceptors for electronic and photonic applications
A technology based on alkyl and atoms, applied in the field of OSC devices, can solve the problems of reduced extinction coefficient, poor short-circuit current density, weakened intramolecular charge transfer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-C8-I
[0216] The synthetic route of embodiment 1-C8-ITCC-Cl
[0217] Step 1: Preparation of 5-chlorothiophene-2-carbonyl chloride
[0218]
[0219] 5-Chlorothiophene-2- carbon Synthesis of acid chlorides (B). To a solution of 5-chlorothiophene-2-carboxylic acid (A, 3.25 g, 20 mmol) in anhydrous dichloromethane (40 mL) was added chlorothiophene (40 mL, 1.0 M in dichloromethane). After stirring the mixture at room temperature overnight, dichloromethane and chlorinated thiophene were evaporated under reduced pressure. The resulting yellow solid was dried under high vacuum and used in the next step without further purification.
[0220] Step 2: Preparation of 2-chloro-4H-cyclopenta[b]thiophene-4,6(5H)-dione
[0221]
[0222] Synthesis of 2-chloro-4H-cyclopenta[b]thiophene-4,6(5H)-dione (C). Add anhydrous aluminum chloride (6.7 g, 50 mmol) and 5-chlorothiophene-2-carbonyl chloride (B) to anhydrous 1,2-dichloroethane (60 mL) under vigorous stirring in an ice bath Malonyl ...
Embodiment 2
[0229] Example 2 - Optical and Electrochemical Properties
Embodiment 2a
[0230] Example 2a: Optical properties
[0231] Thin film UV-Vis absorption spectra of the polymer from Example 2 were obtained on a Perkin Elmer Lambda 20 UV / VIS spectrophotometer. All film samples were spin-cast on ITO / ZnO substrates. The solution UV-Vis absorption spectrum under high temperature is collected on Perkin Elmer Lambda950UV / VIS / NIR spectrophotometer. The temperature of the cuvettes was controlled using a Perkin Elmer PTP 6+6 Peltier System supplied by a Perkin ElmerPCB 1500 Water Peltier System. Before each measurement, the system was kept at the target temperature for at least 10 min to reach thermal equilibrium. Stopped cuvettes (Sigma Z600628) were used to avoid volatilization during the measurement. The onset of absorption was used to estimate the polymer bandgap.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com