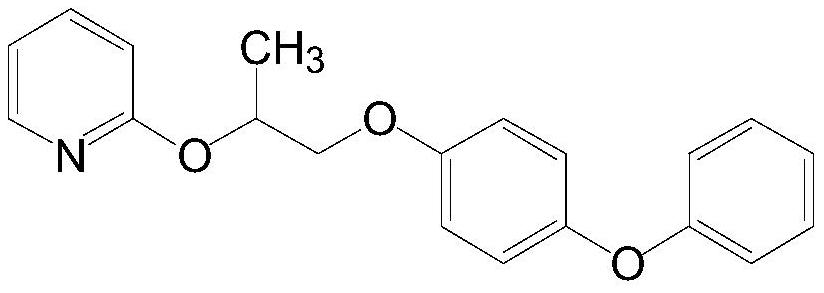
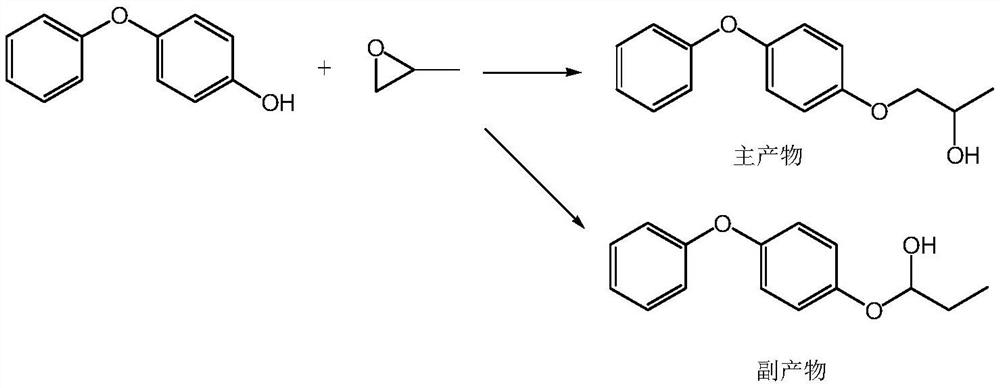
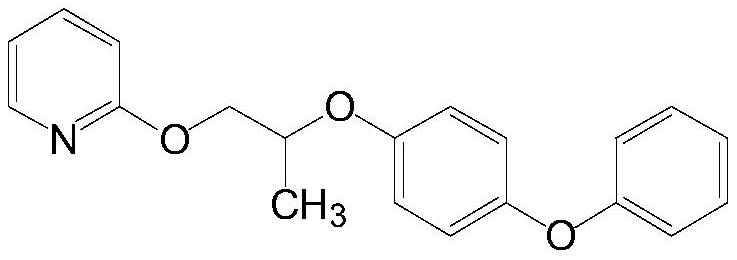
Purification method of pyriproxyfen technical, product obtained by purification method and application of product
A purification method, the technology of pyriproxyfen, which is applied in the direction of organic chemistry, can solve the problems of high content of isomer impurities, insufficient product purity, low overall yield, etc., and achieve the effects of ensuring purity, facilitating precipitation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Purification method of pyriproxyfen crude product:
[0061] (1) Add 100 g of crude pyriproxyfen technical product with a purity of 87.5% into a flask containing 90 mL of xylene, then add 100 mL of tetrahydrofuran, heat to reflux at 45° C., filter to remove insoluble impurities, and obtain solution A;
[0062] (2) Cool the solution A obtained in step (1) to 25°C at a cooling rate of 2°C / min, then place it in an ice-water bath to continue cooling and crystallization until the crystals are completely precipitated, filter, and dry under reduced pressure to obtain Purified pyriproxyfen technical substance.
[0063] In this embodiment, 86 g of white crystals were purified, and through quantitative analysis by high performance liquid chromatography, the purity of the obtained purified pyriproxyfen technical substance was 98.7%, and the yield of the purification process was 97%.
Embodiment 2
[0065] Purification method of pyriproxyfen crude product:
[0066] (1) Add 100 g of crude pyriproxyfen technical product with a purity of 93% into a flask containing 90 mL of n-hexane, then add 100 mL of methanol, heat to reflux at 45° C., filter to remove insoluble impurities, and obtain solution A;
[0067] (2) Cool the solution A obtained in step (1) to 25°C at a cooling rate of 3°C / min, add 3g of pure pyriproxyfen, and then place it in an ice-water bath to continue cooling and crystallization until the crystals are completely precipitated, Filter and dry under reduced pressure to obtain the purified pyriproxyfen technical substance.
[0068] Purified in this example, 92.4 g of white crystals were obtained. Quantitative analysis by high performance liquid chromatography showed that the purity of the purified pyriproxyfen technical product was 99.2%, and the yield of the purification process was 98.5%.
Embodiment 3
[0070] In this example, the cooling rate of step (2) in Example 1 is replaced by 3.5° C. / min, and other conditions are completely the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com