Molecular targeting drug-entrapped liposome and application of liposome in preparation of drug for treating tumors
A molecular targeting drug and targeting liposome technology, applied in the field of biomedicine, can solve problems such as affecting bioavailability, affecting curative effect, poor solubility, etc. effect, the effect of increasing flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
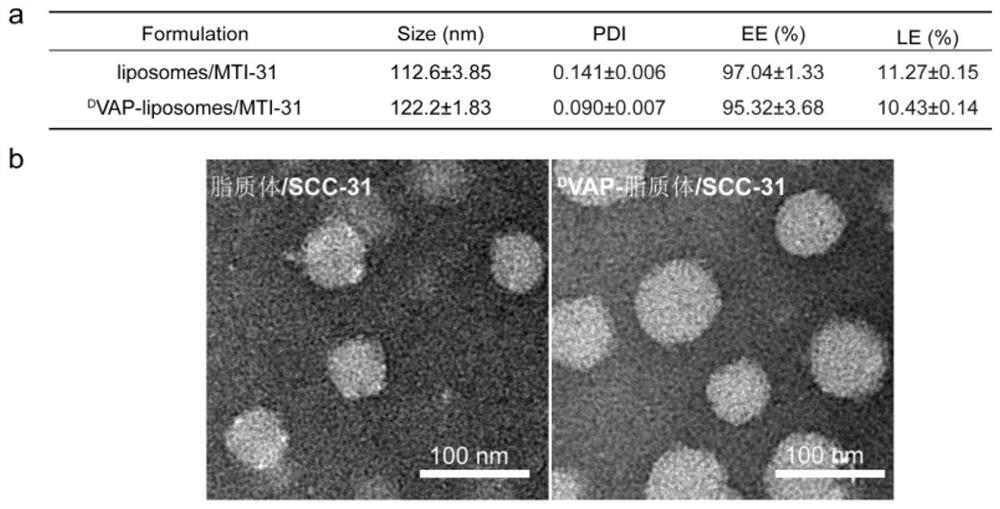
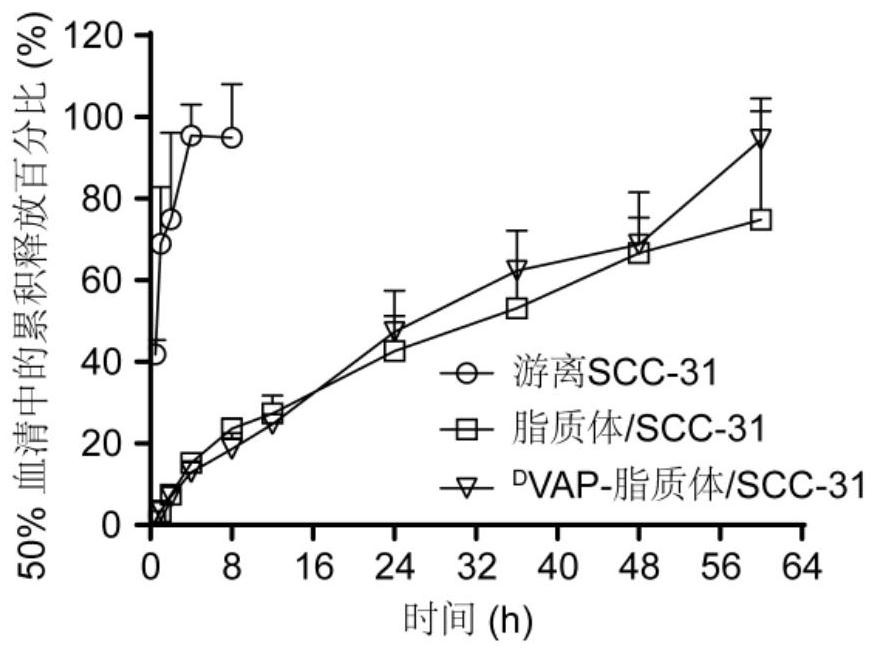
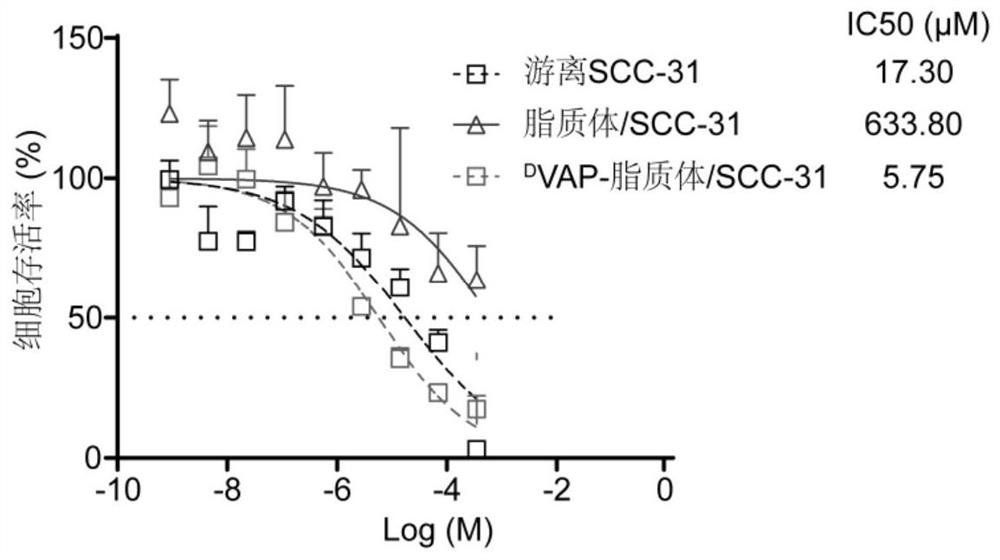
[0047] Embodiment 1, preparation and characterization of liposome / SCC-31 by active drug loading method
[0048] This example discloses the preparation and characterization of liposome / molecular targeting drug SCC-31 by the active drug loading method, which specifically includes the following steps:
[0049] The membrane material formulation of liposome / SCC-31 is HSPC / Chol / mPEG2000-DSPE (50:45:5, molar ratio). The above membrane materials were weighed and dissolved in chloroform, and the organic solvent was removed by rotary evaporation under reduced pressure to obtain a uniform lipid membrane, which was dried in vacuum for 24 hours. Add a certain volume of 0.2M citric acid solution, and shake in a water bath at 60°C for 2 hours to obtain a liposome suspension. Ultrasonic cell pulverizer ultrasonicated for 10min (80w, ultrasonic 2s, interval 1s), and blank liposomes were obtained. Physiological saline was eluted and the blank liposome extracorporeal aqueous phase was replaced...
Embodiment 2
[0050] Embodiment 2, active drug loading method preparation D VAP-liposome / SCC-31 and its characterization
[0051] This example discloses the preparation of active drug loading method D VAP-liposome / molecular targeting drug SCC-31 and its characterization, specifically include the following steps:
[0052] D The formulation of VAP-liposome / SCC-31 membrane material is HSPC / Chol / mPEG 2000 -DSPE / D VAP-PEG 3400 - DSPE (50:45:3:2, molar ratio). The above membrane materials were weighed and dissolved in chloroform, and the organic solvent was removed by rotary evaporation under reduced pressure to obtain a uniform lipid membrane, which was dried in vacuum for 24 hours. Add a certain volume of 0.2M citric acid solution, and shake in a water bath at 60°C for 2 hours to obtain a liposome suspension. Ultrasonic cell pulverizer ultrasonicated for 10min (80w, ultrasonic 2s, interval 1s), and blank liposomes were obtained. Physiological saline was eluted and the blank liposome ex...
Embodiment 3
[0053] Embodiment 3, preparation and characterization of liposome / SCC-31 by passive drug loading method
[0054] This example discloses the preparation and characterization of liposome / molecular targeting drug SCC-31 by passive drug loading method, which specifically includes the following steps:
[0055] The membrane material formulation of liposome / SCC-31 is HSPC / Chol / mPEG 2000 - DSPE (50:45:5, molar ratio), respectively weigh the above membrane materials and dissolve them in ethanol, and inject them dropwise into 0.9% normal saline under the condition of magnetic stirring. Ultrasonic cell pulverizer ultrasonicated for 10min (80w, ultrasonic 2s, interval 1s) to obtain liposome / SCC-31. The particle diameter of the liposome / SCC-31 measured by a Malvern laser scattering particle size analyzer was 124.27 nm, and the PDI was 0.201. The encapsulation efficiency of liposome measured by HPLC was 85.56%, the drug loading capacity was 8.91%, and the drug concentration contained in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com