Simultaneous reaction and separation of chemicals
A reactor, gas-phase reaction technology, applied in chemical instruments and methods, chemical/physical processes, inorganic chemistry, etc., can solve problems such as low methane conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] In a first example, methane is generated according to the simplified schematic Figure 16 into hydrogen and carbon in a reactor configuration. Methane was bubbled together with argon 1601 into molten metal alloy 1603 at 1065°C consisting of Ni / Bi in a molar ratio of 0.27:0.73, generating hydrogen and carbon. Referring to Table 1, when catalytic molten metal was used, almost complete conversion to hydrogen with high selectivity to hydrogen demonstrated the successful application of molten metal in methane pyrolysis.
[0122] The melt was prepared as follows: Solid bismuth and nickel were mixed in a Ni / Bi molar ratio of 0.27:0.73 in a 3 cm x 1.2 m stainless steel tube closed at one end with a weld cap. The reactor was mounted in a 4 cm alumina sleeve surrounded by four 30.5 cm 850 watt ceramic heaters surrounded by ceramic insulation. The space between the alumina tube and the stainless steel reactor is purged with nitrogen to prevent the stainless steel from scaling du...
Embodiment 2
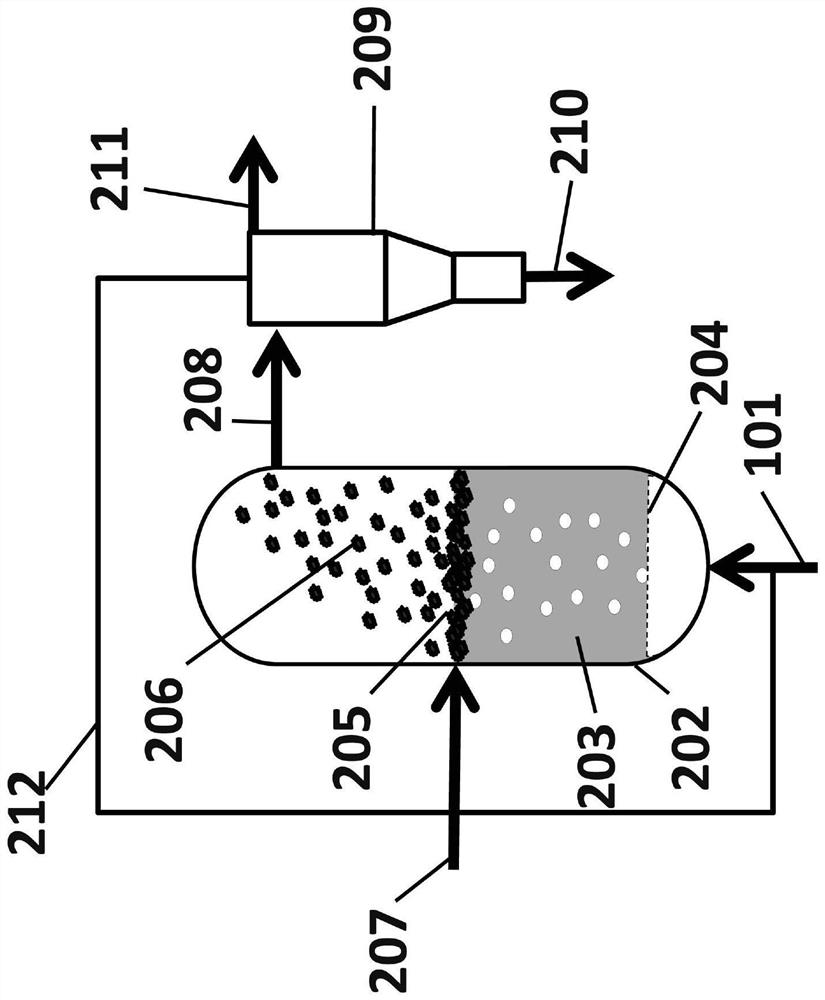
[0127] In a second example, methane is generated according to the simplified schematic Figure 16 into hydrogen and carbon, among other products, in a reactor configuration. The reactor was placed so that the temperature of the headspace was lower than that of the melt. Methane was bubbled together with argon 1601) through a tube 1602 inserted into a molten metal alloy 1603 consisting of nickel / bismuth at 1050°C in a molar ratio of 0.27:0.73 in the first run, and in the second run The molten metal alloy 1603 in the test consisted of pure bismuth at 1050°C. The bubble column reactor was constructed of quartz and connected to a mass spectrometer to analyze the products 1605. The molten metal tower 1604 has a height of 150 mm and a diameter of 12 mm. A 3 mm quartz tube down into the liquid metal 1602 was used to introduce the gas into the melt. With the Swagelok UltraTorr Tube Fitting, the depth of the tube can be controlled to any height. All liquids are heated with an 850 ...
Embodiment 3
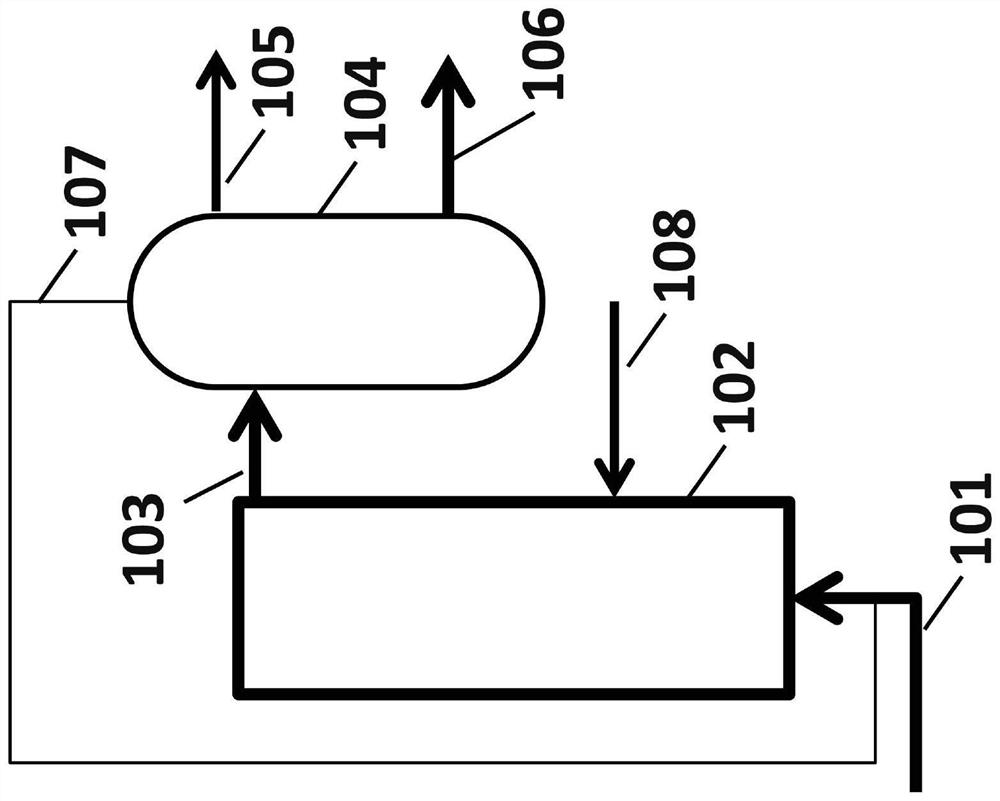
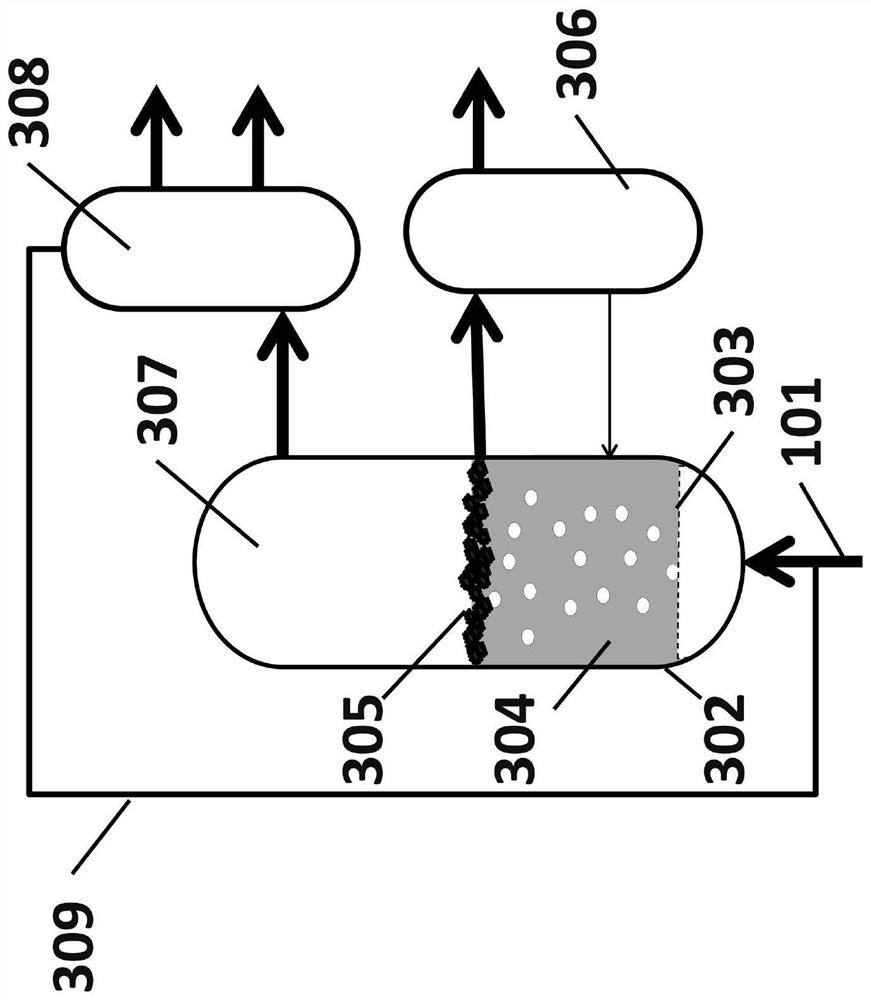
[0132] In a third embodiment, methane is Figure 10 into hydrogen and carbon in a reactor configuration. At position 1002, methane was bubbled with argon into a molten metal alloy at 1050°C consisting of Ni / Bi in a molar ratio of 0.27:0.73, and hydrogen and carbon were produced. All unreacted methane leaves the reactor as a gas at position 1004 along with some hydrogen. Some carbon is deposited at location 1005 , some carbon dissolves into the melt 1003 and diffuses to a separate location and deposits at location 1006 . In addition, some hydrogen dissolves and diffuses in the melt and exits the reactor at 1007, separated from unreacted methane.
[0133] The methane conversion and hydrogen yield from outlet gas stream 1004 for this experiment are shown in Table 3 below. During the pyrolysis in this example, methane conversion was observed to remain stable for 170 hours, supporting the conclusion that the solid carbon formed moves to the surface of the molten metal, thereby p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com