Patents
Literature
78results about "High temperature liquid-gas reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
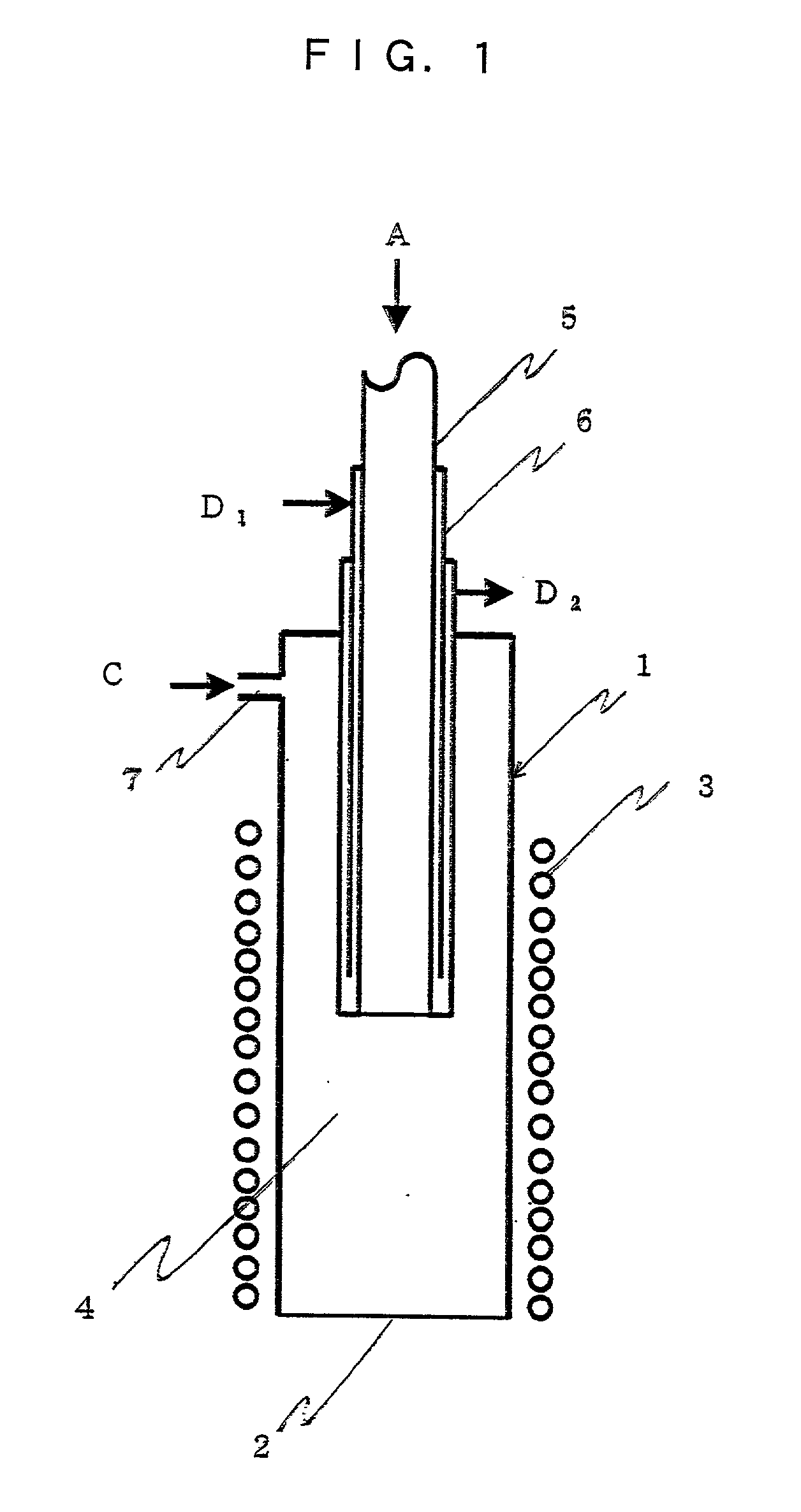
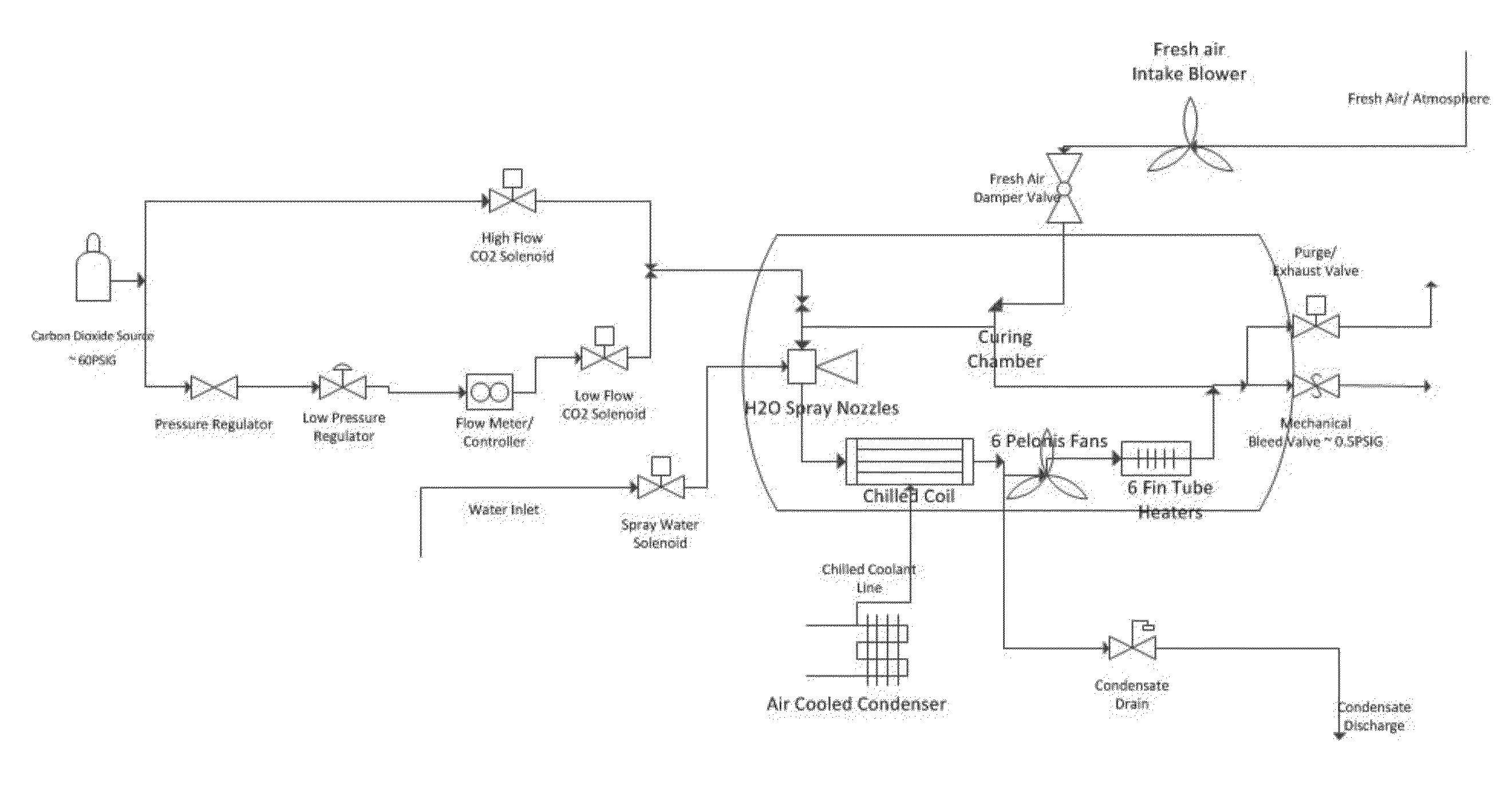
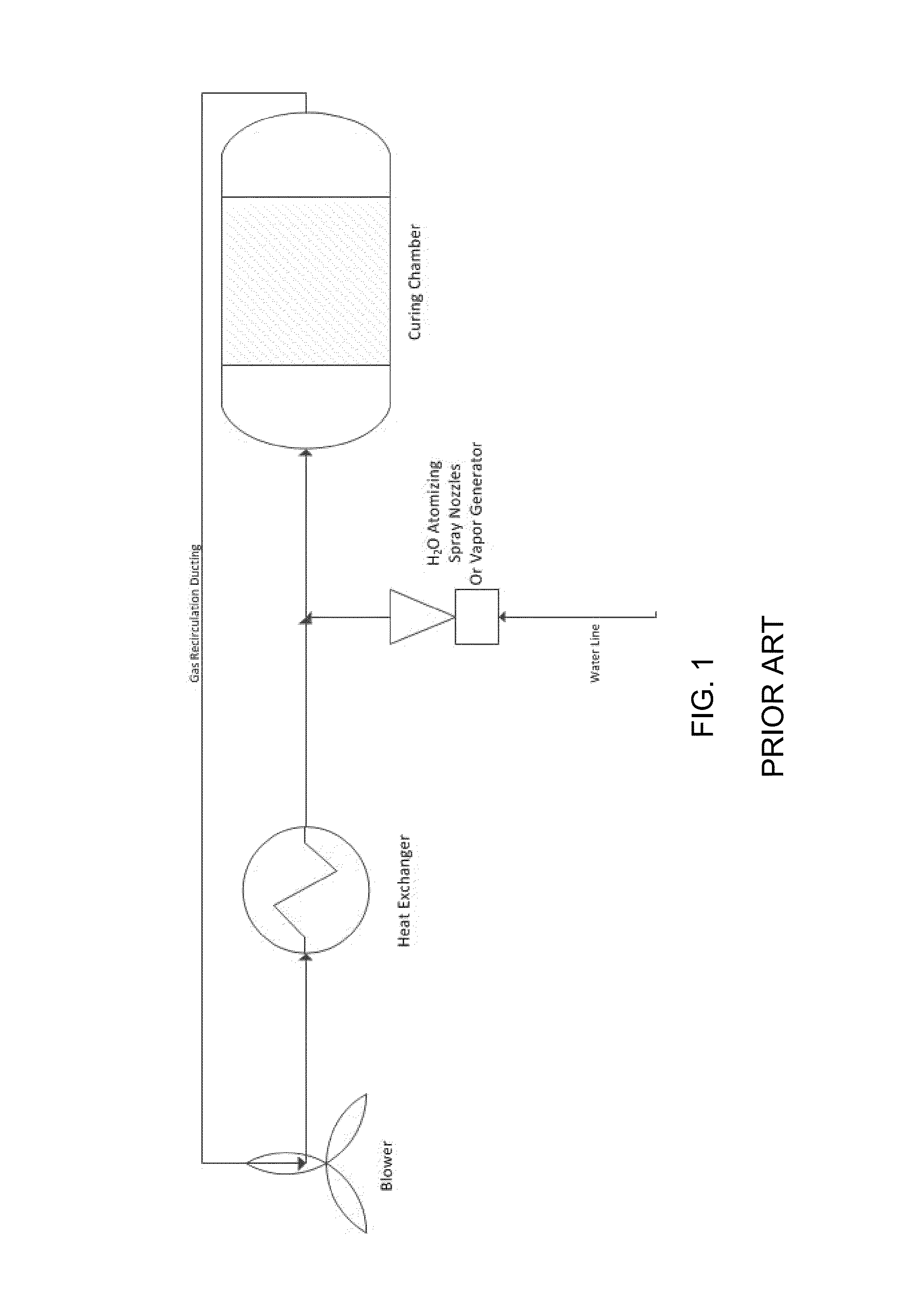
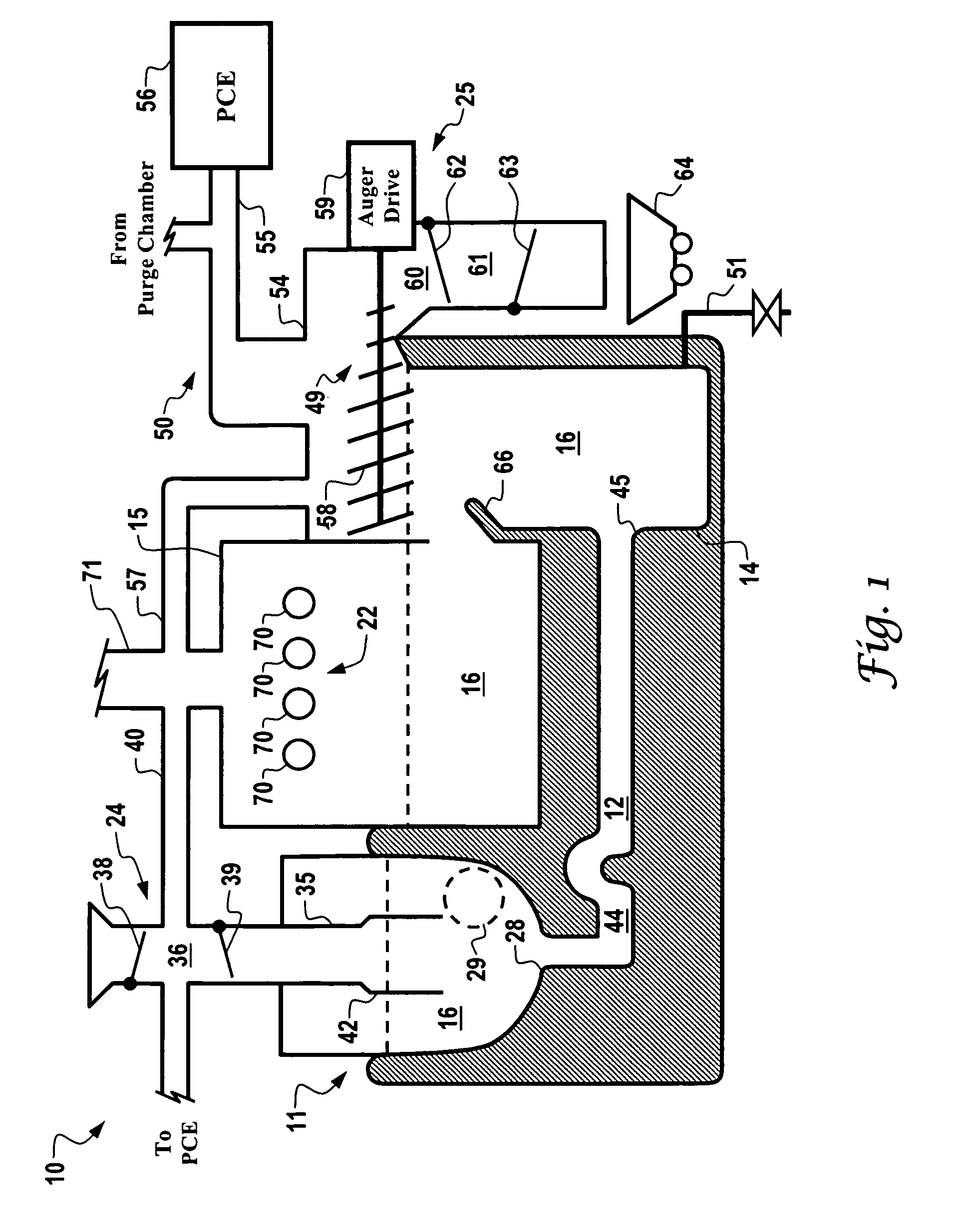
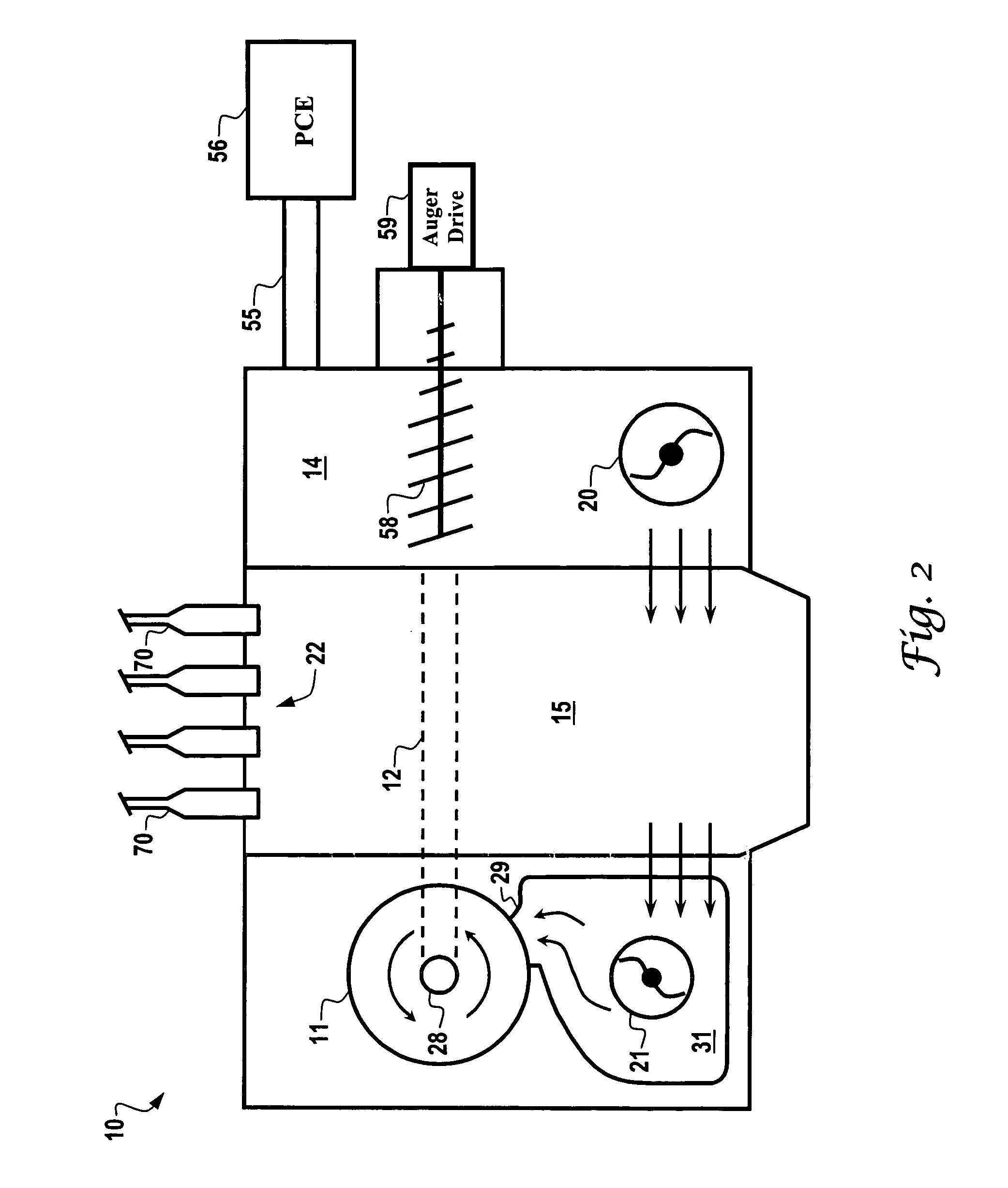
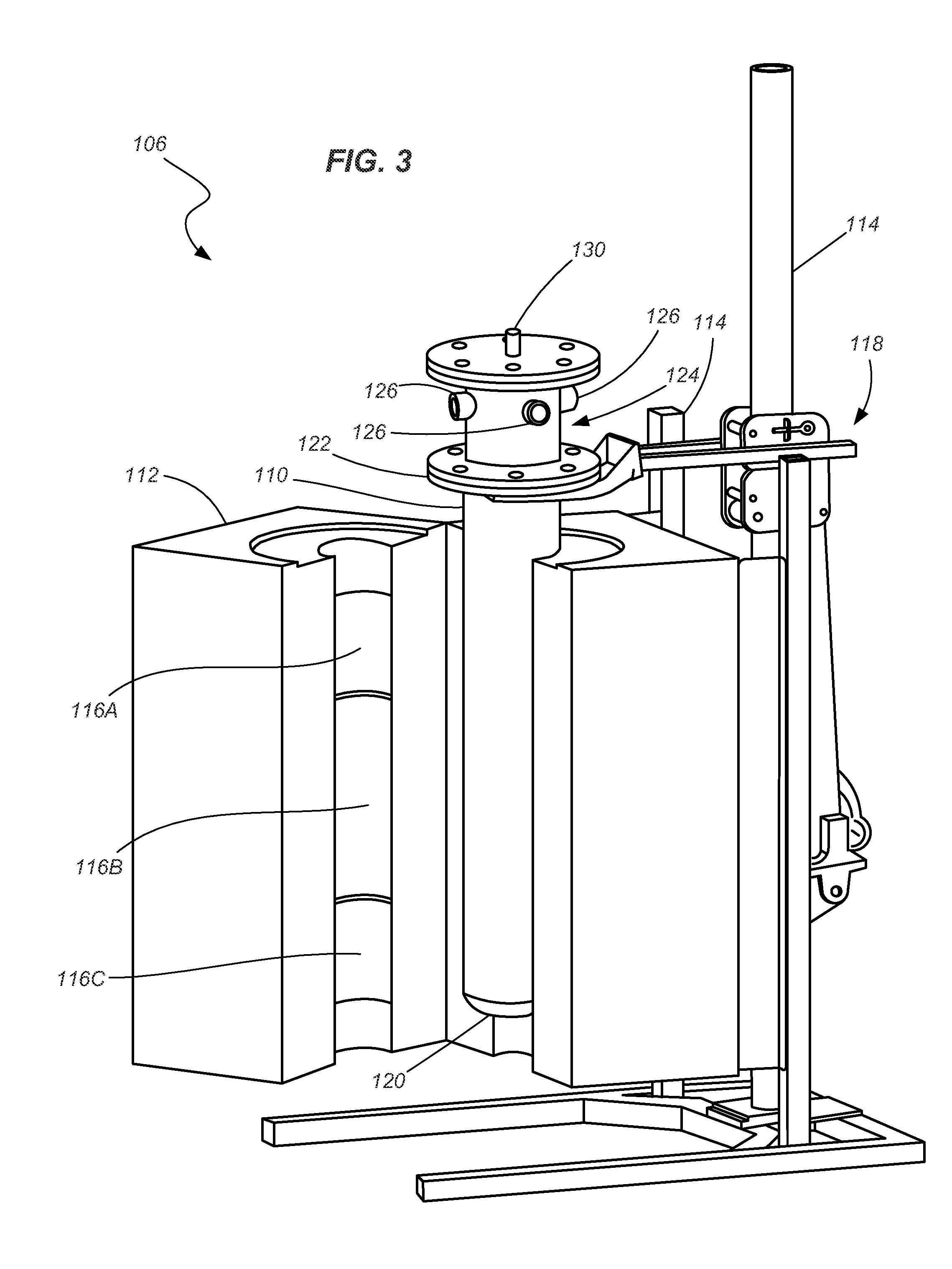
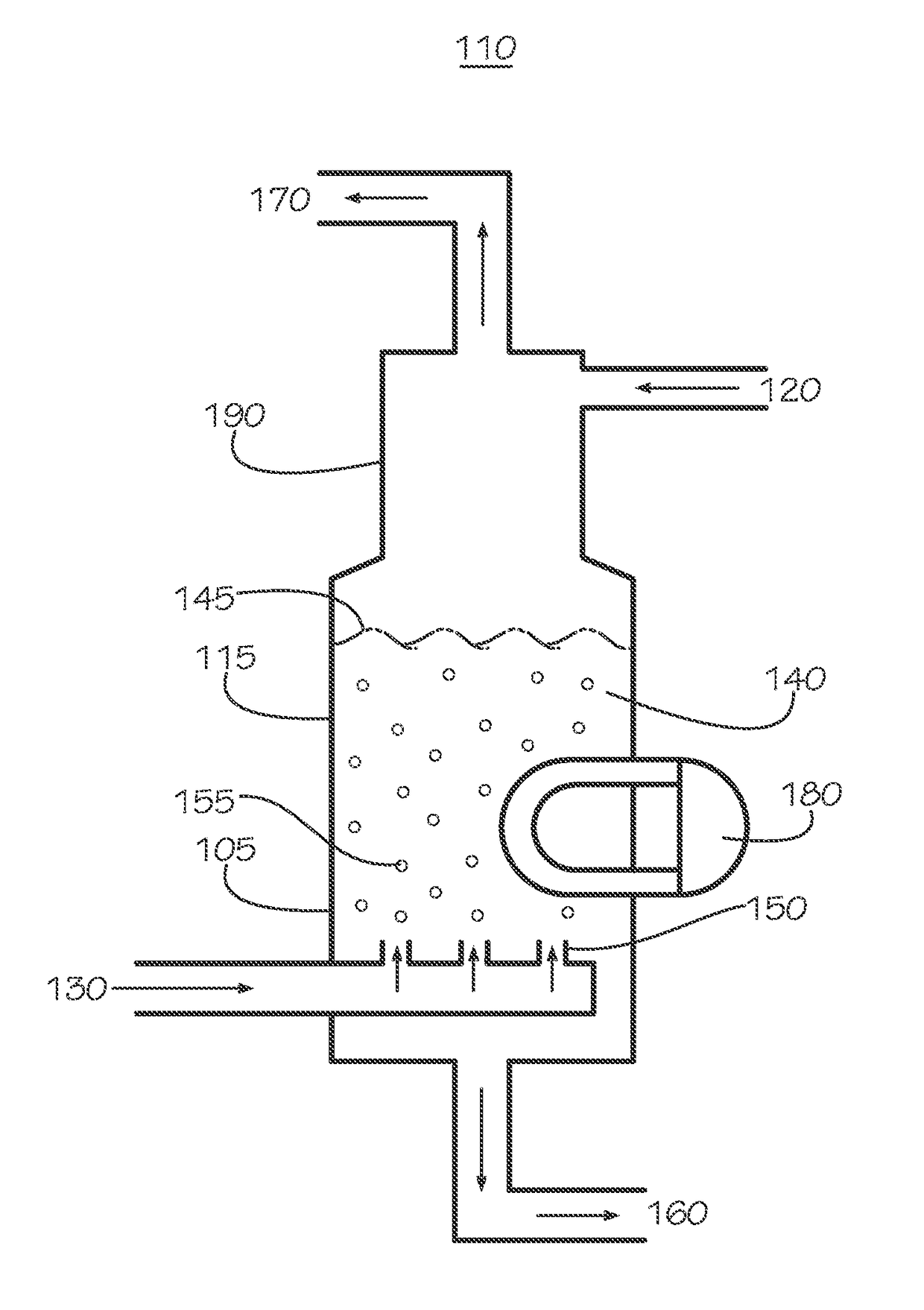
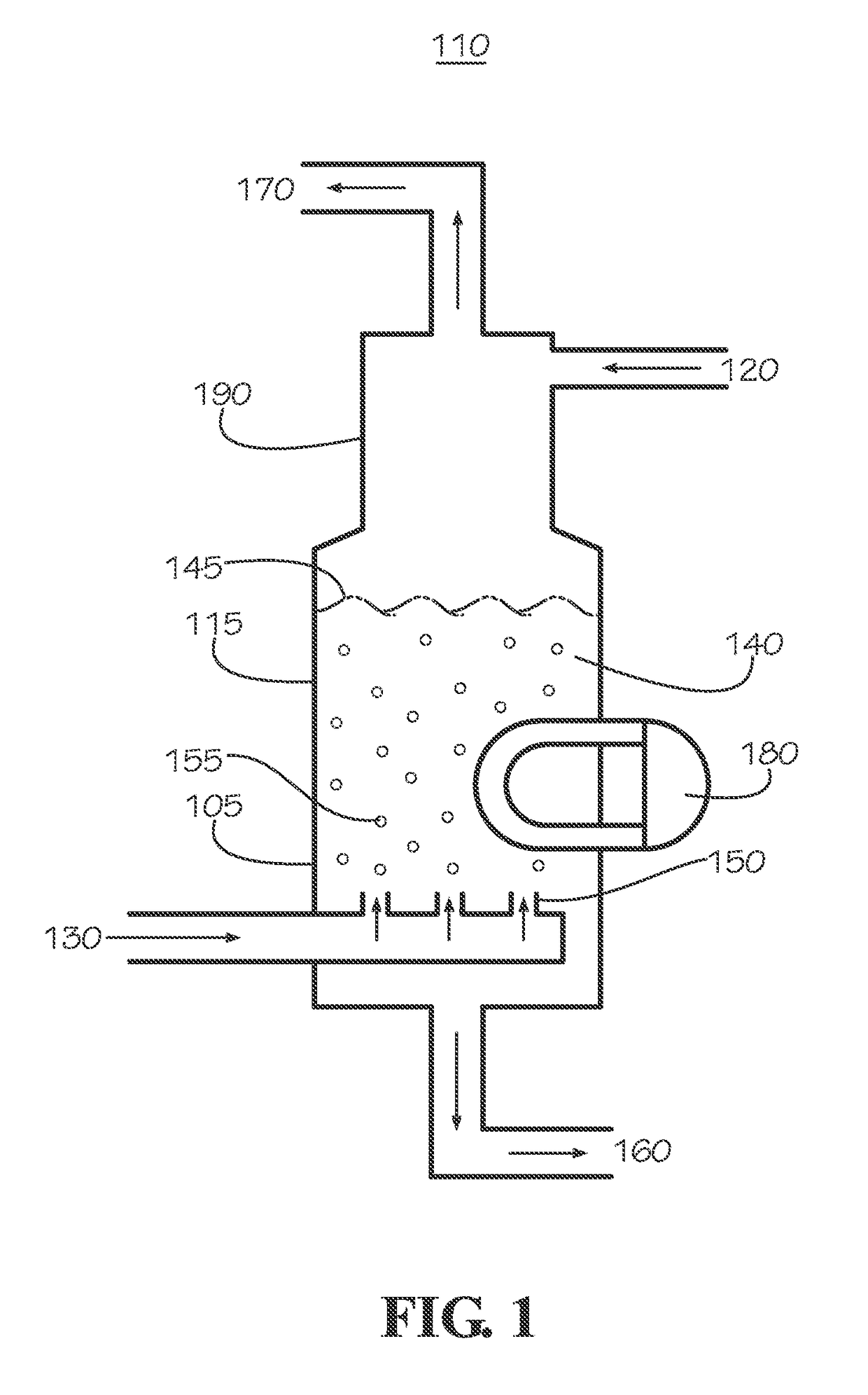
Curing systems for materials that consume carbon dioxide and method of use thereof
ActiveUS20140322083A1Low costIncrease consumptionMuffle furnacesTemperatue controlProcess engineeringProduct gas
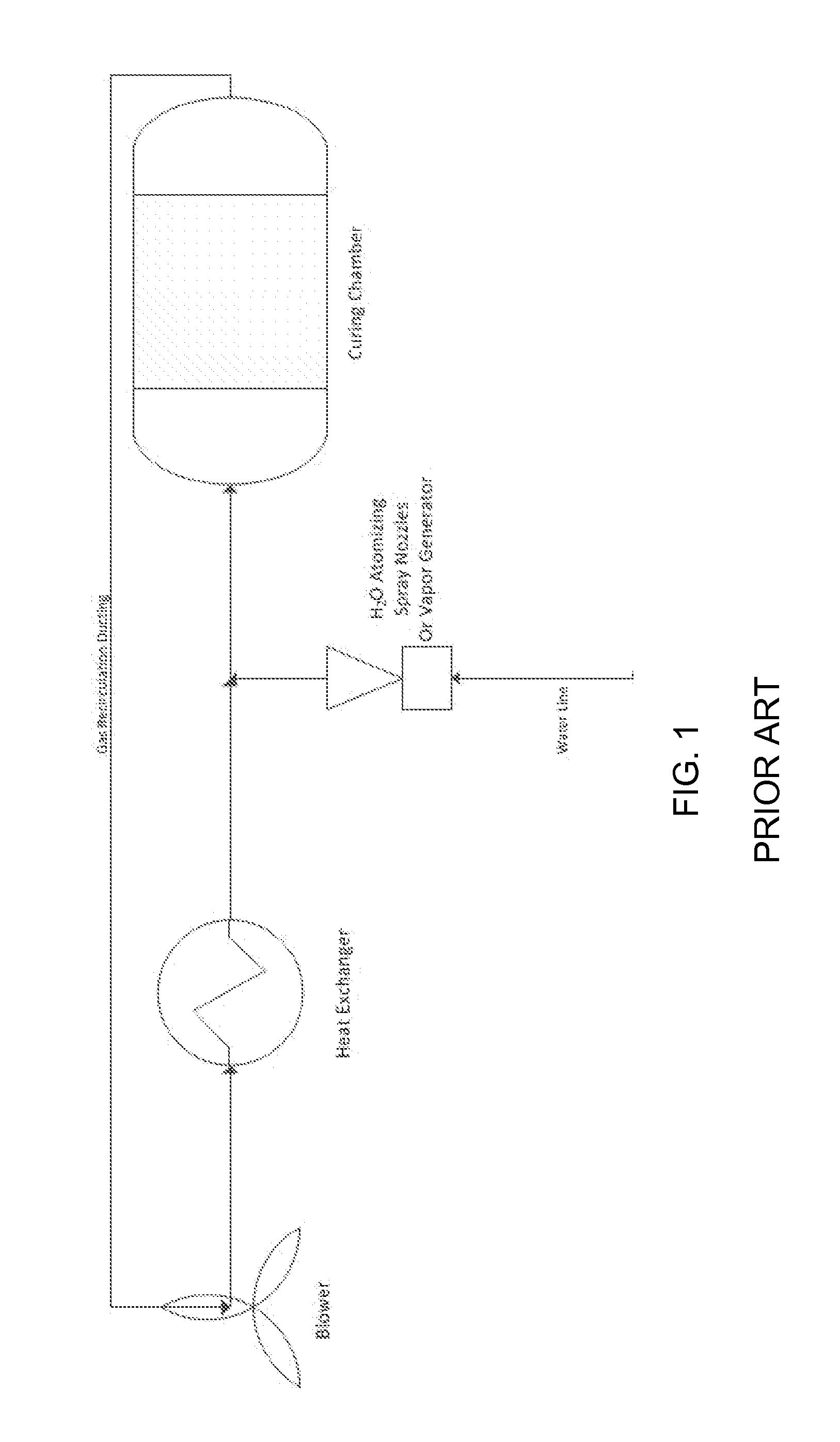
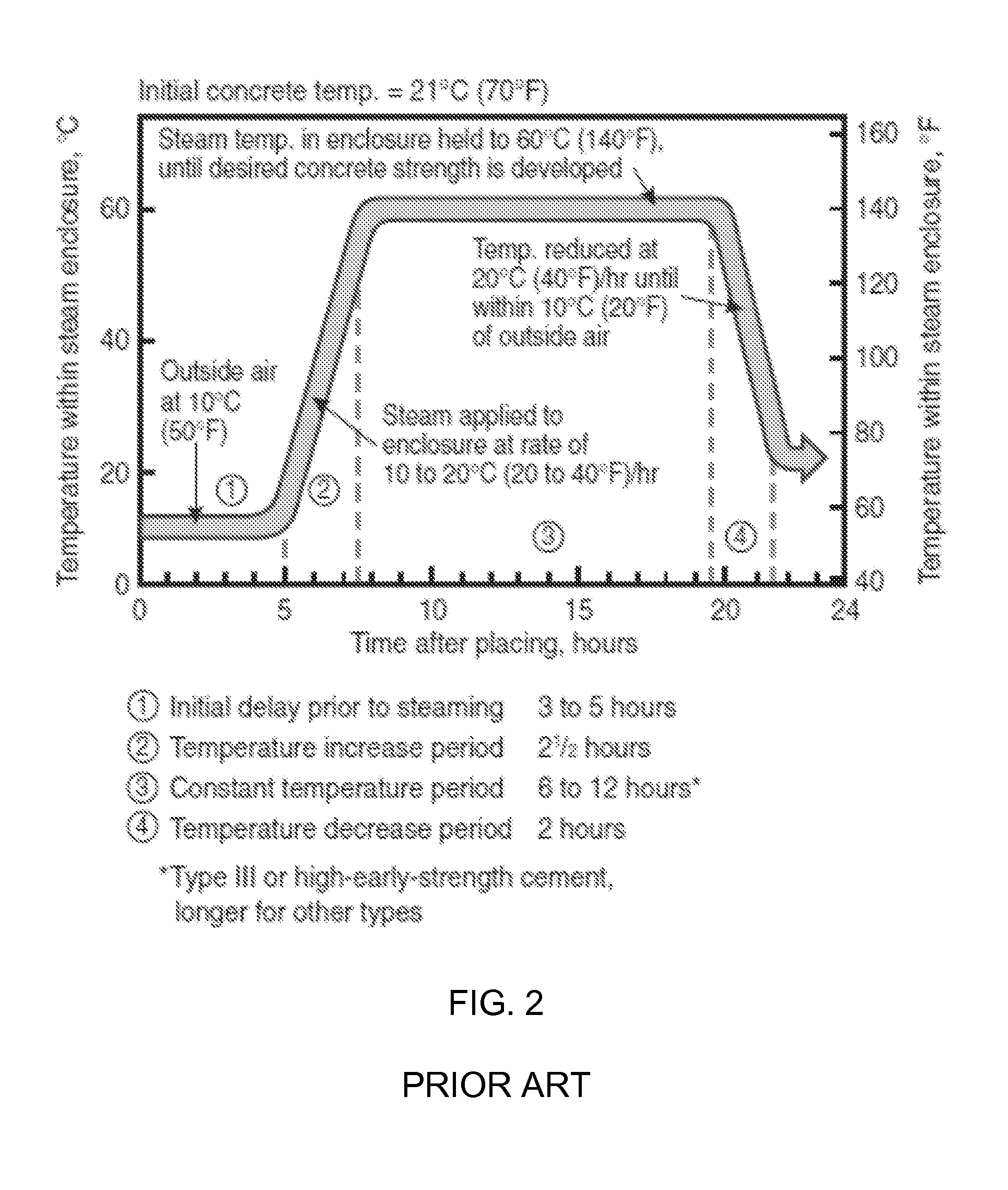
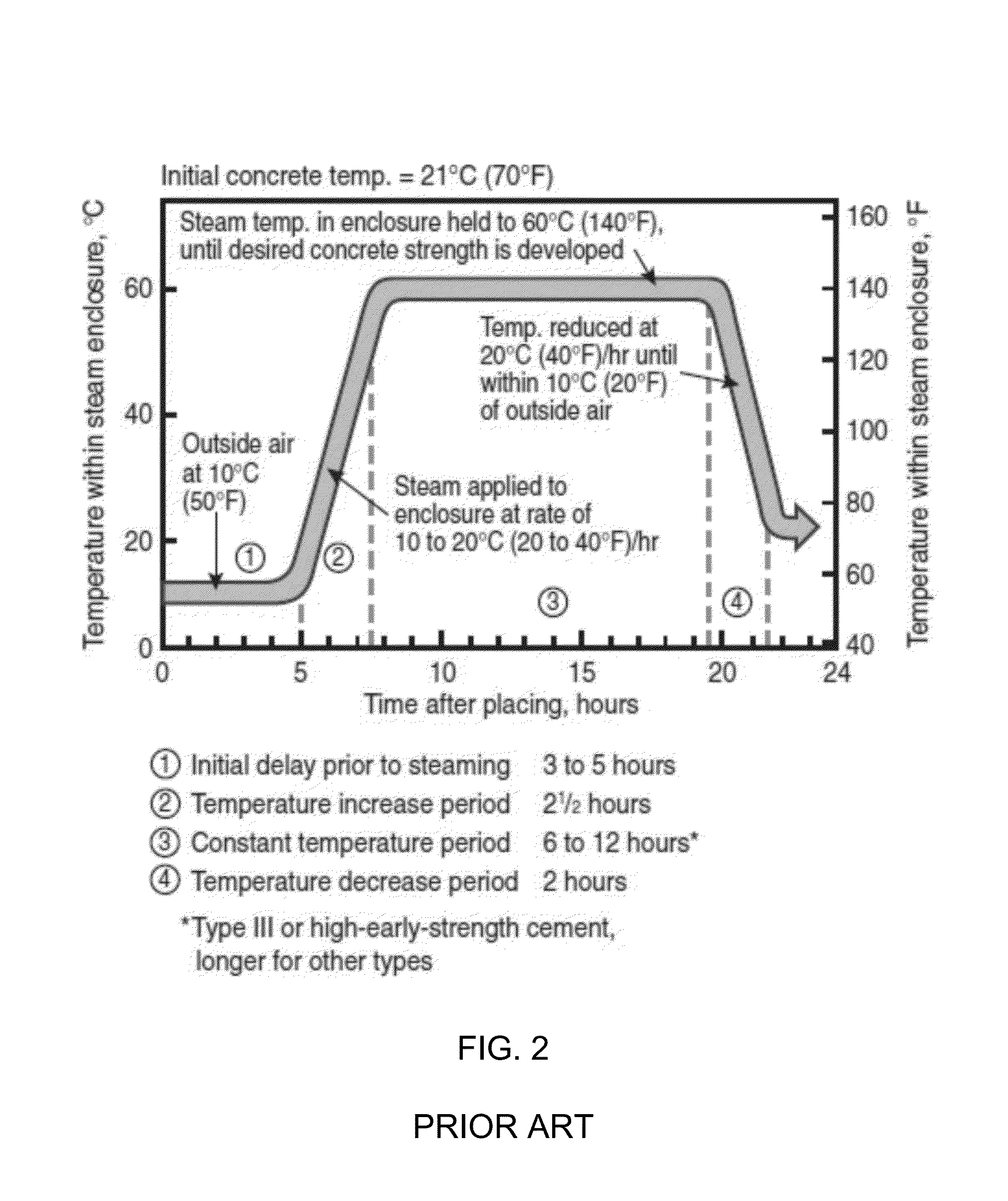
The invention provides a curing system that is useful for curing materials that consume carbon dioxide as a reagent. The system has a curing chamber that contains the material to be cured and a gas that contains carbon dioxide. The system includes apparatus that can deliver carbon dioxide to displace ambient air upon loading the system, that can provide carbon dioxide as it is needed and as it is consumed, that can control carbon dioxide concentration, temperature and humidity in the curing chamber during the curing cycle and that can record and display to a user the variables that occur during the curing process.
Owner:SOLIDIA TECH
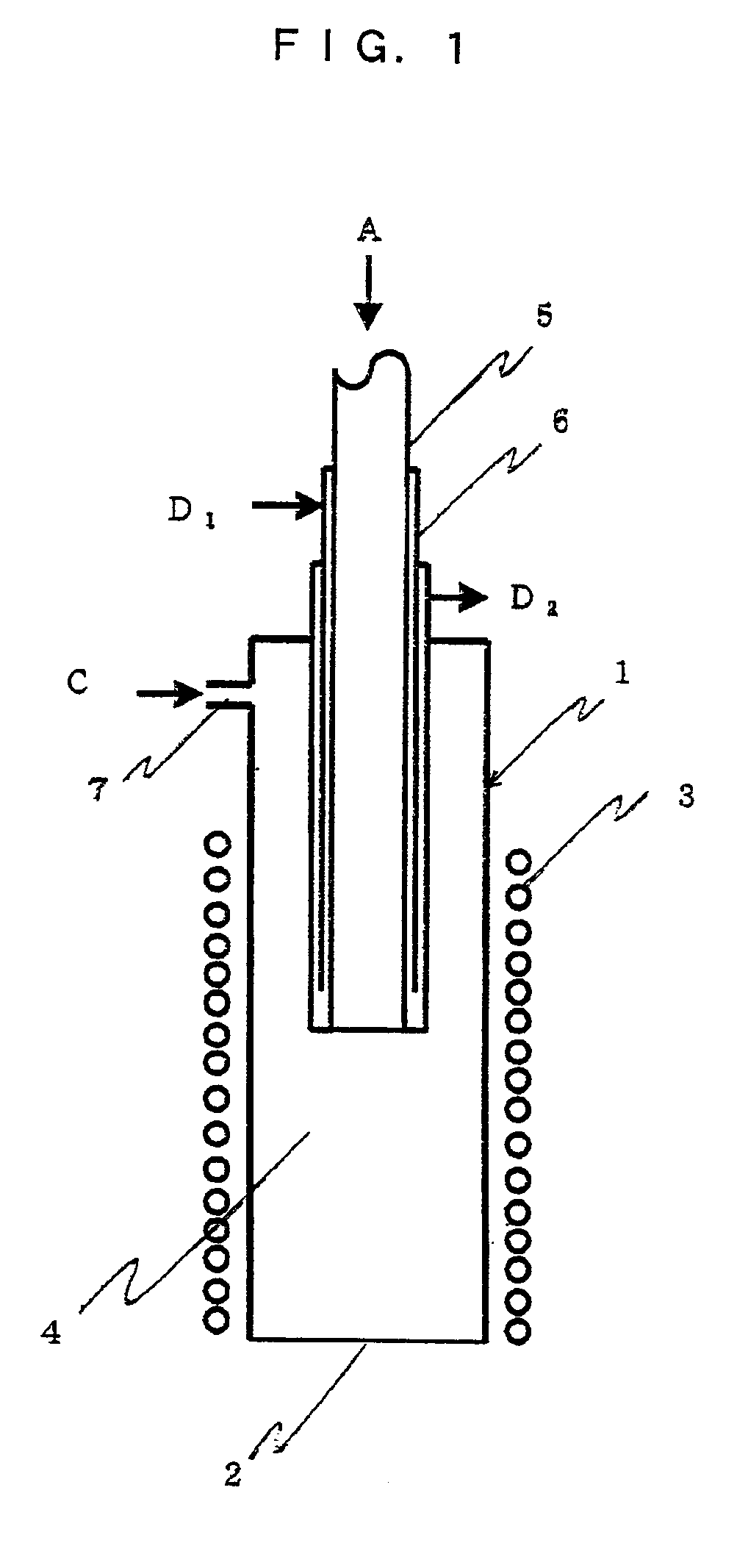
Polycrystalline silicon and process and apparatus for producing the same
InactiveUS20020104474A1Improve the heating effectSuitable structureFrom solid stateSemiconductor/solid-state device manufacturingChlorosilanePolycrystalline silicon
Foamed polycrystalline silicon having bubbles therein and an apparent density of 2.20 g / cm3 or less. This silicon generates an extremely small amount of fine grains by crushing and can be easily crushed. There is also provided a method of producing foamed polycrystalline silicon. There is further provided a polycrystalline silicon production apparatus in which the deposition and melting of silicon are carried out on the inner surface of a cylindrical vessel, a chlorosilane feed pipe is inserted into the cylindrical vessel to a silicon molten liquid, and seal gas is supplied into a space between the cylindrical vessel and the chlorosilane feed pipe.
Owner:TOKUYAMA CORP
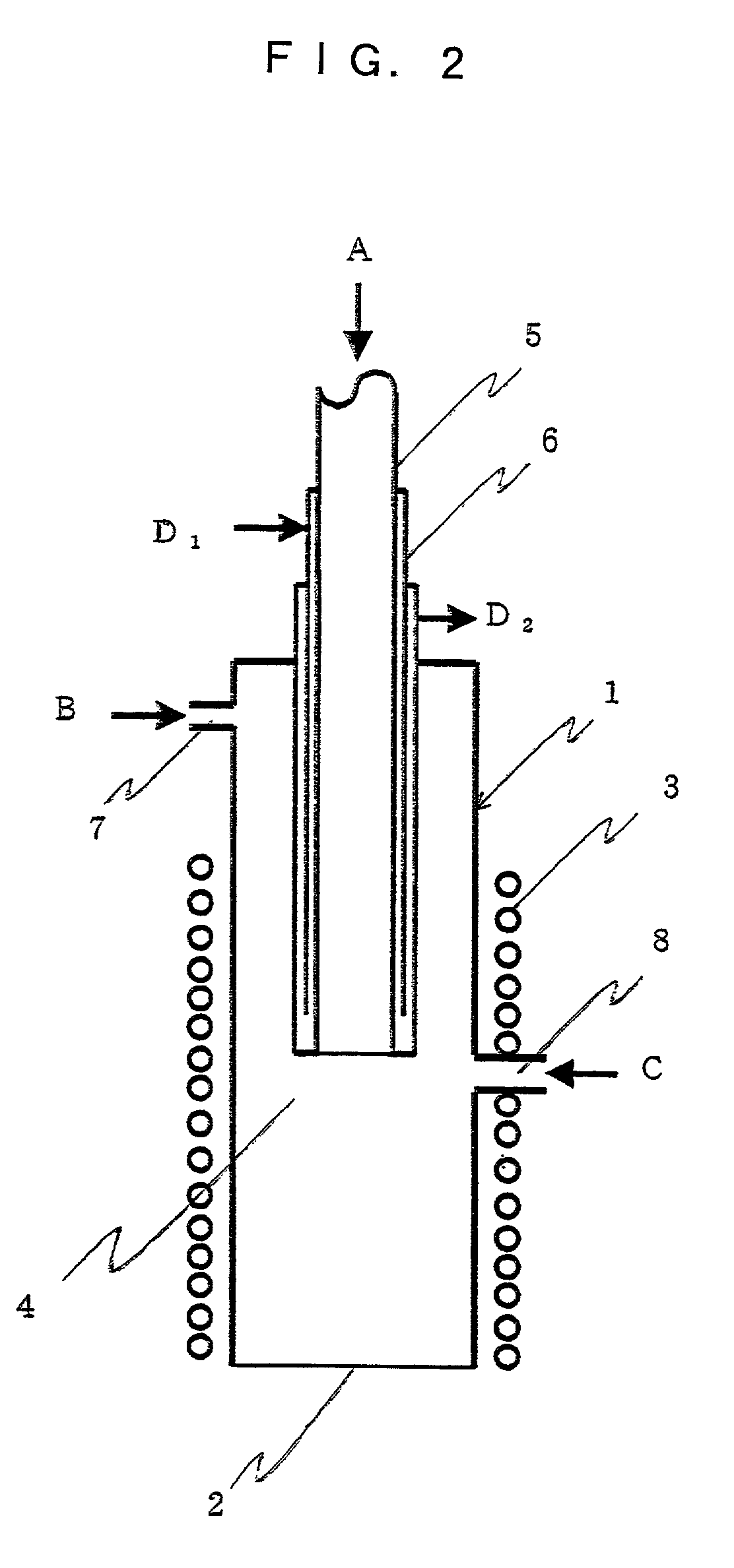
Curing systems for materials that consume carbon dioxide and method of use thereof
ActiveUS9221027B2Low costIncrease consumptionMuffle furnacesDrying gas arrangementsProcess engineeringAmbient air
The invention provides a curing system that is useful for curing materials that consume carbon dioxide as a reagent. The system has a curing chamber that contains the material to be cured and a gas that contains carbon dioxide. The system includes apparatus that can deliver carbon dioxide to displace ambient air upon loading the system, that can provide carbon dioxide as it is needed and as it is consumed, that can control carbon dioxide concentration, temperature and humidity in the curing chamber during the curing cycle and that can record and display to a user the variables that occur during the curing process.
Owner:SOLIDIA TECH
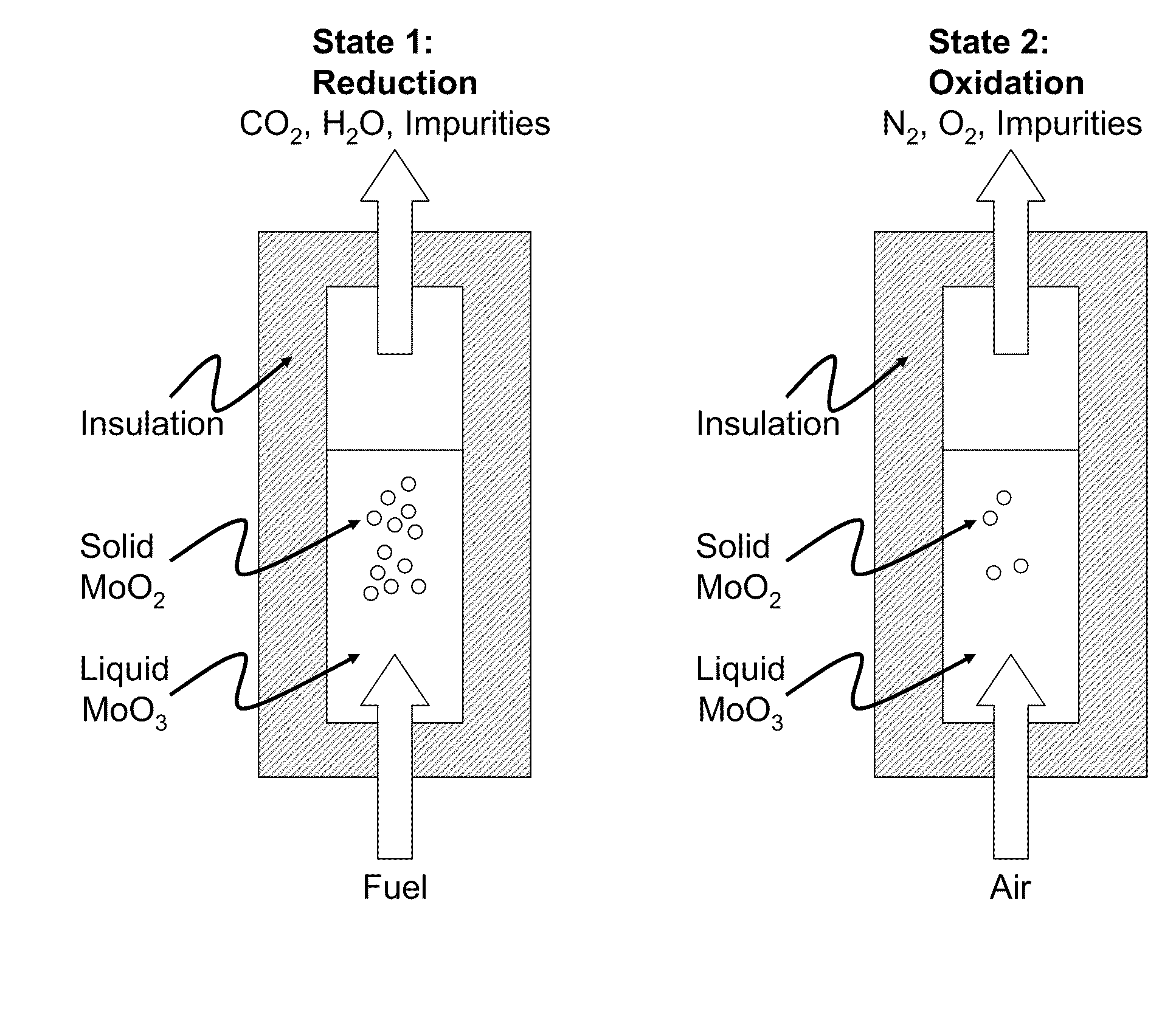
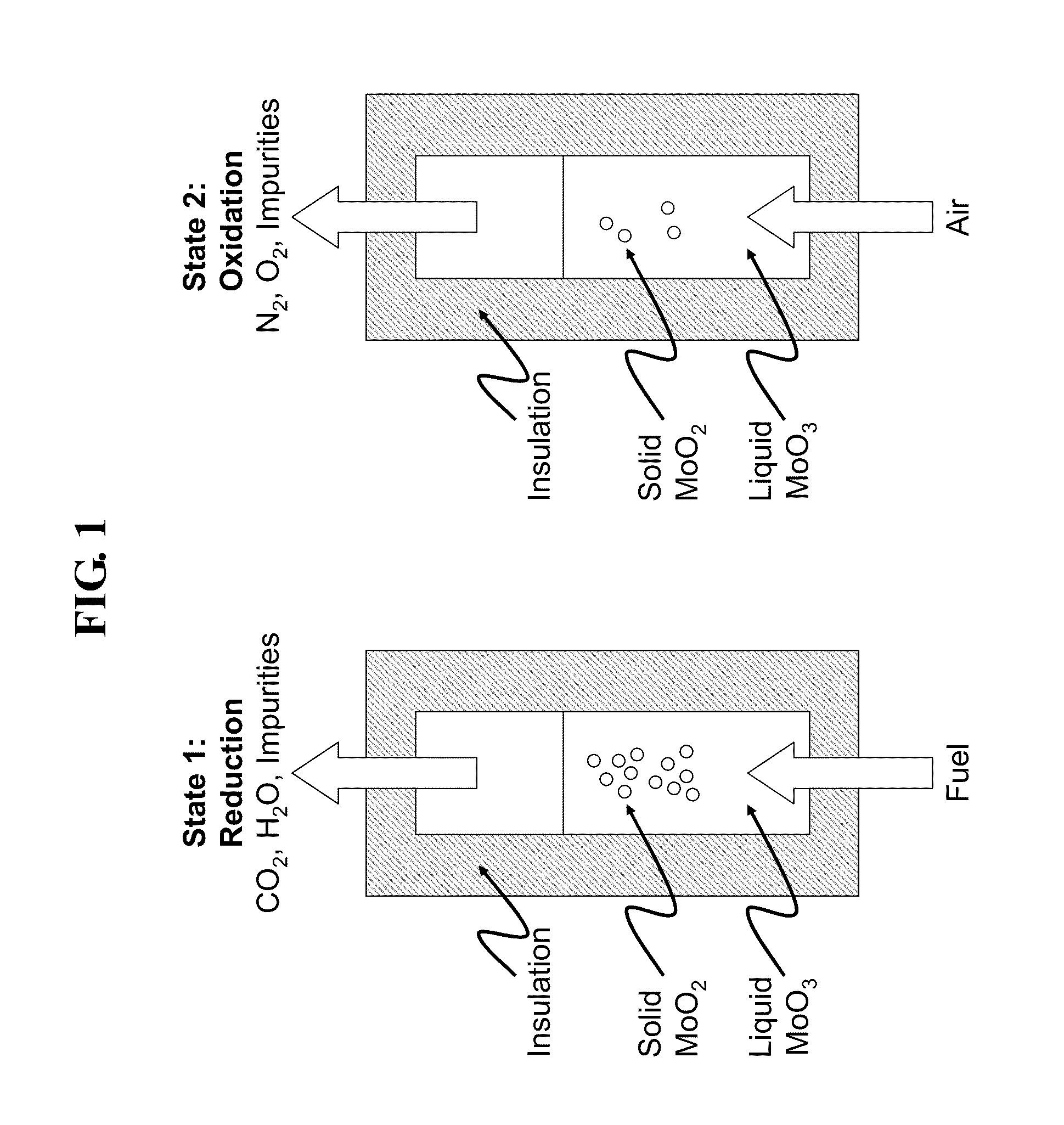
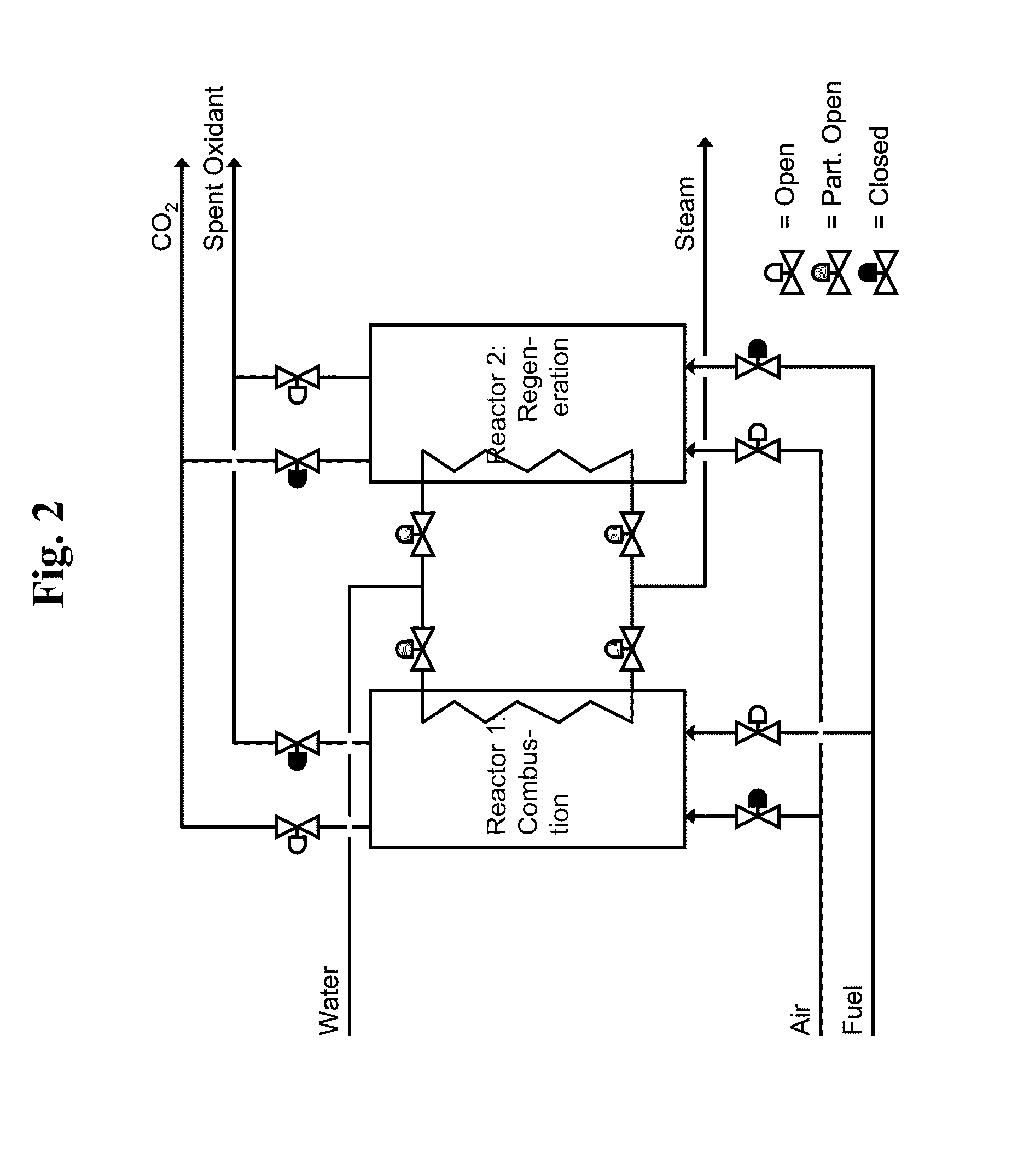
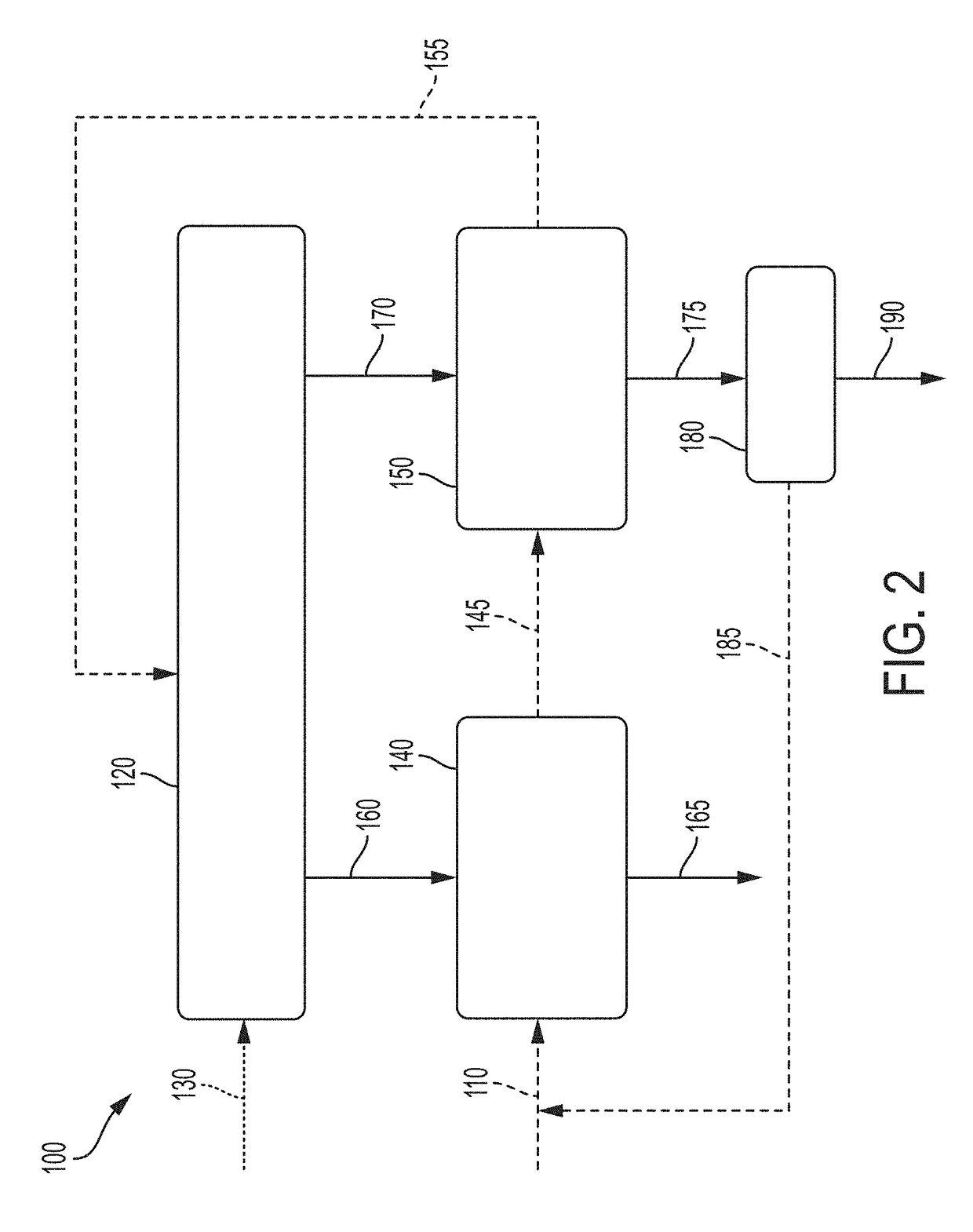
Liquid-phase chemical looping energy generator
Owner:PHILLIPS 66 CO
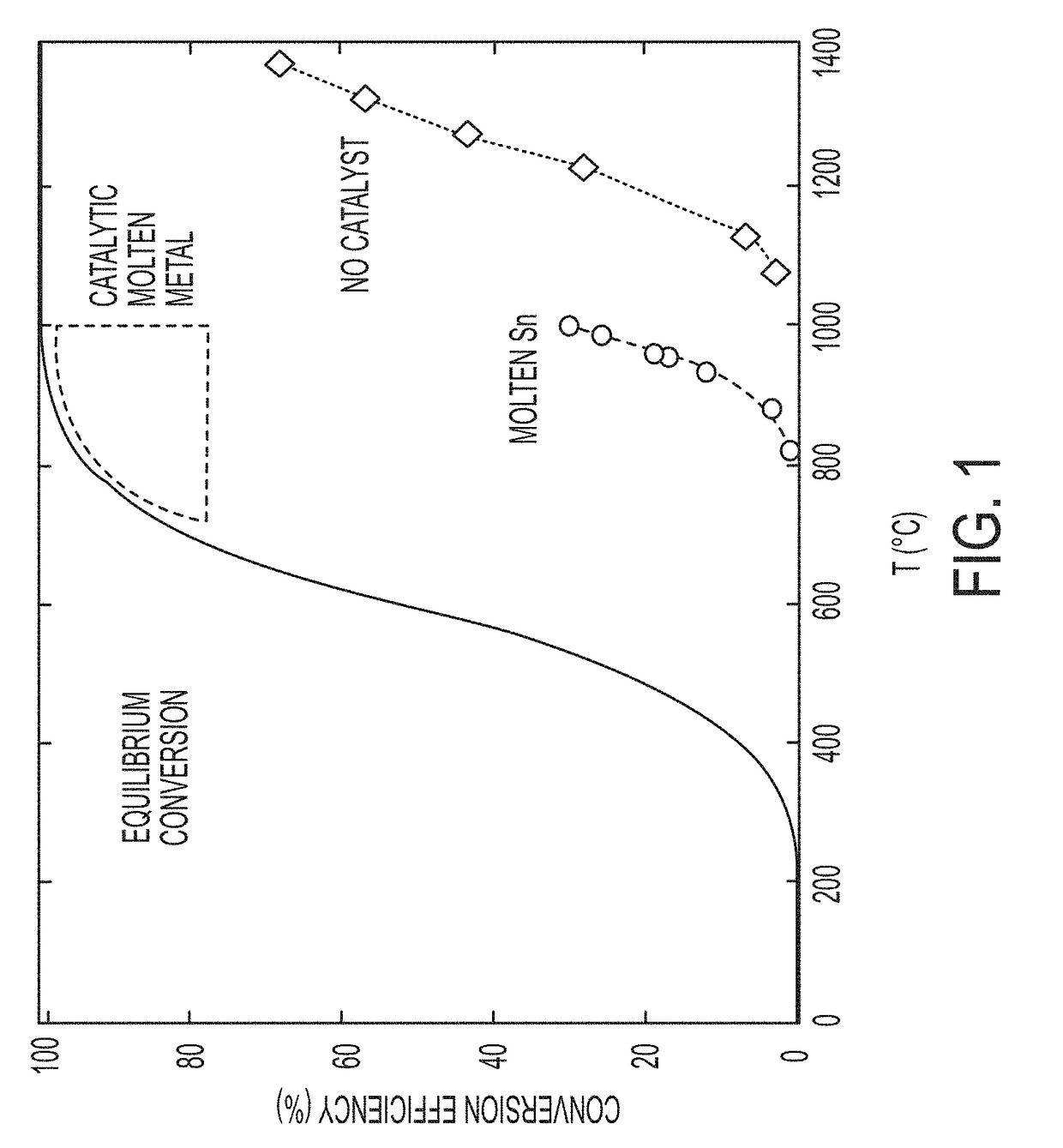
System and method for pyrolysis using a liquid metal catalyst
ActiveUS20190055173A1Elongated bubble shapeLarge specific surface areaPigmenting treatmentGraphiteSolid carbonHydrogen
A process for decomposing a hydrocarbon-containing composition includes feeding the hydrocarbon-containing composition to a reactor containing a catalytically active molten metal or a catalytically active molten metal alloy, wherein the metal or alloy catalyzes a decomposition reaction of the hydrocarbon-containing composition into a hydrogen-rich gas phase and a solid carbon phase. The solid carbon phase is insoluble in the metal or alloy. The process may be a continuous process.
Owner:XEROX CORP
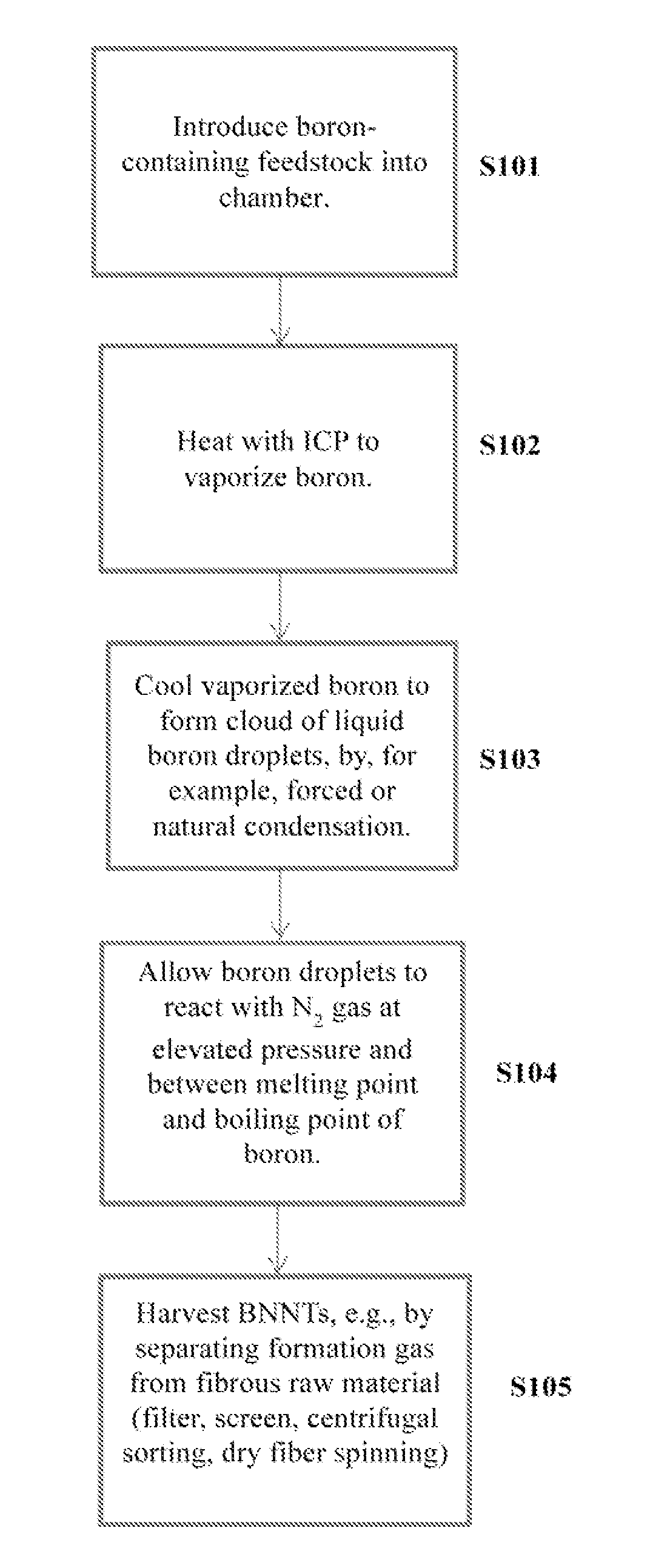
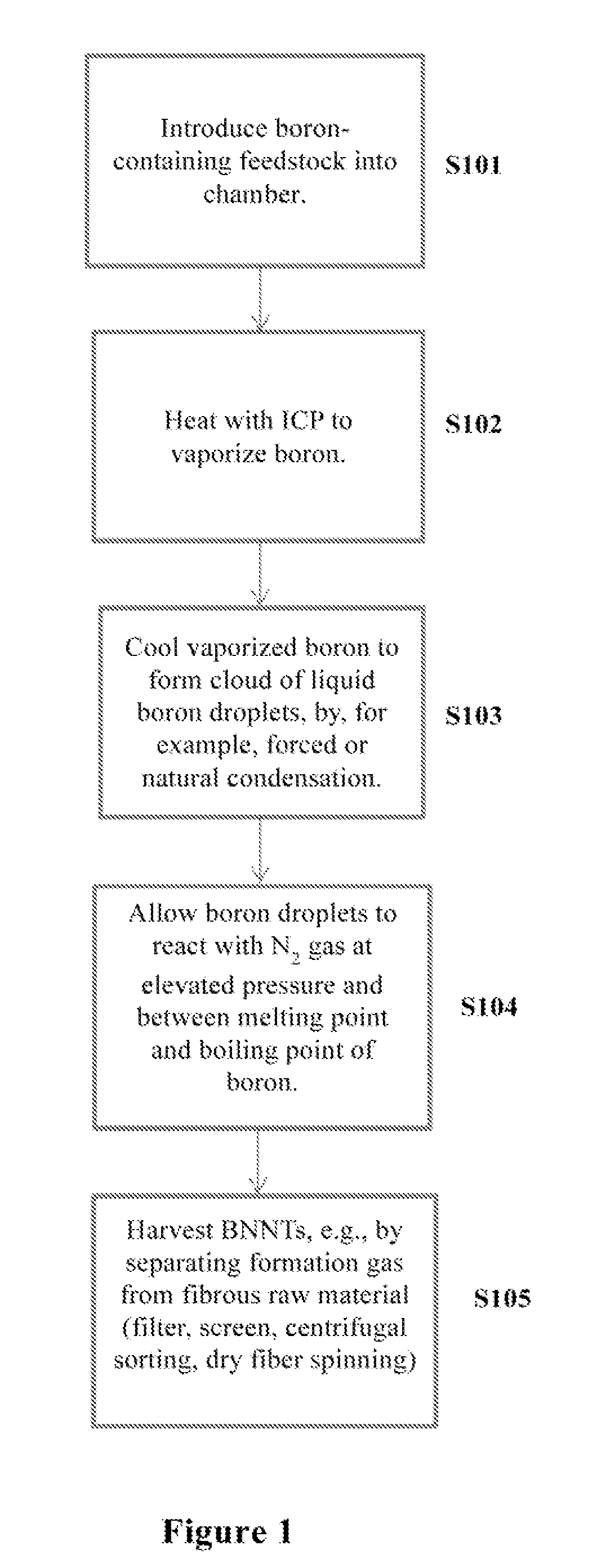
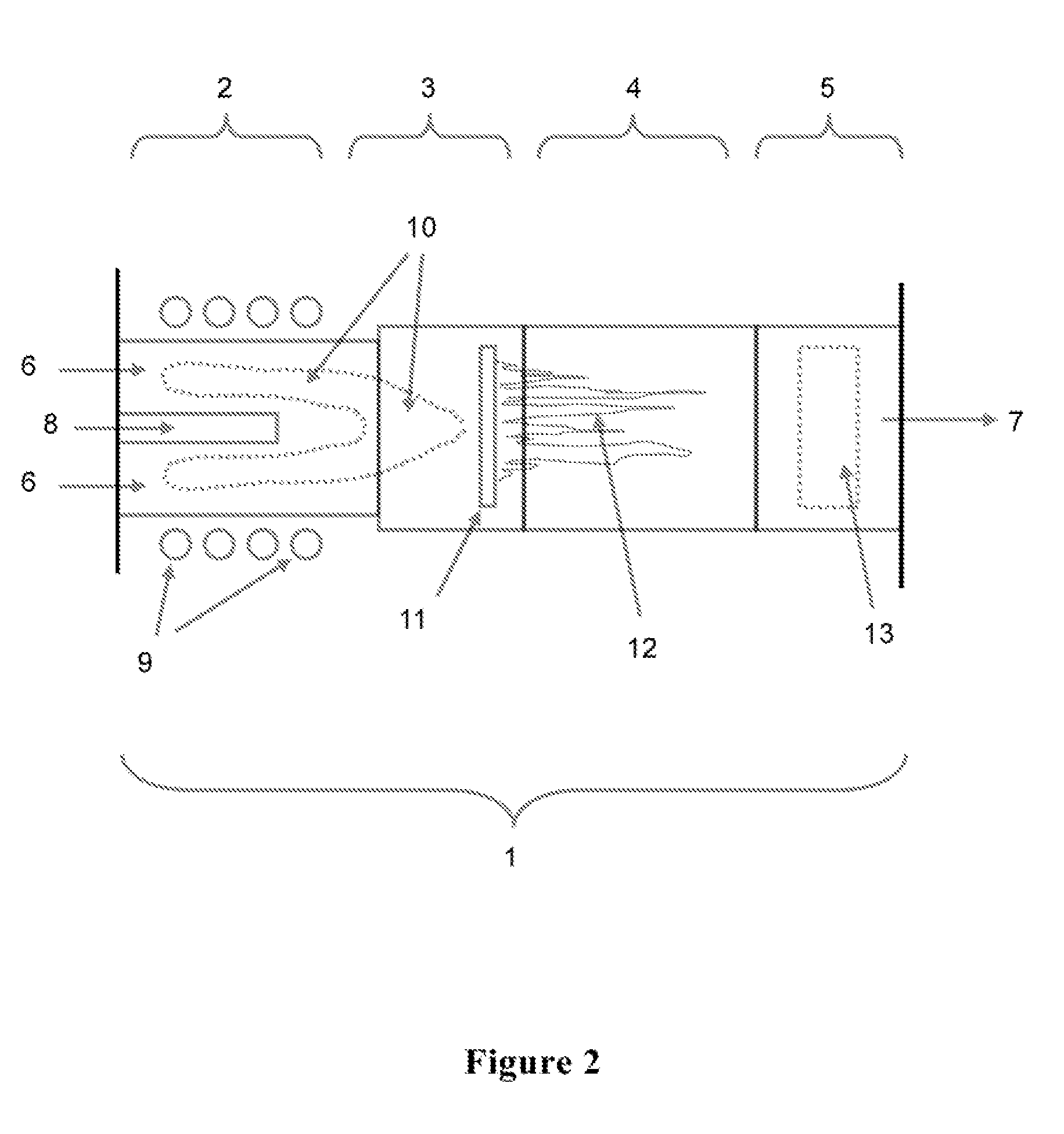
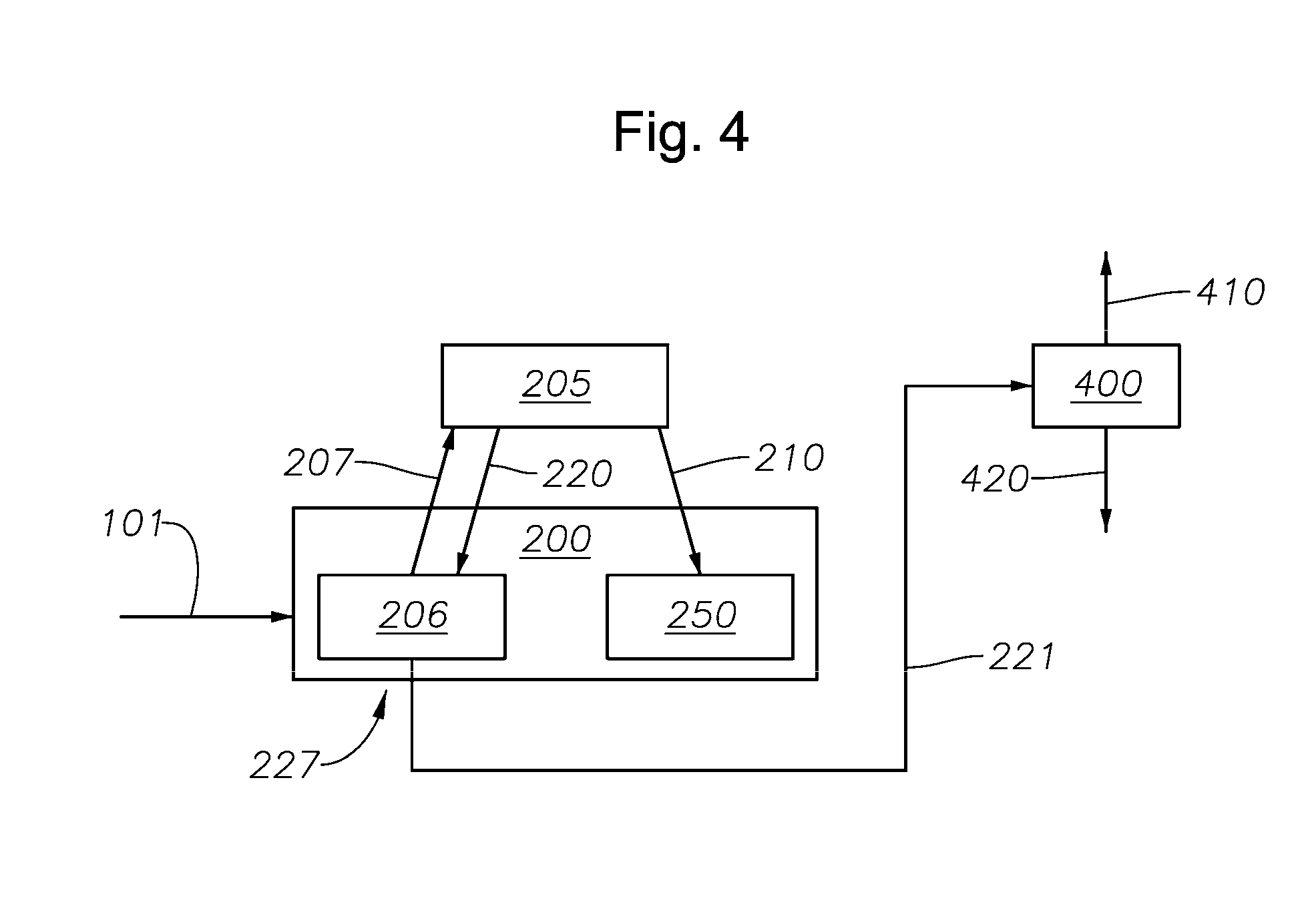
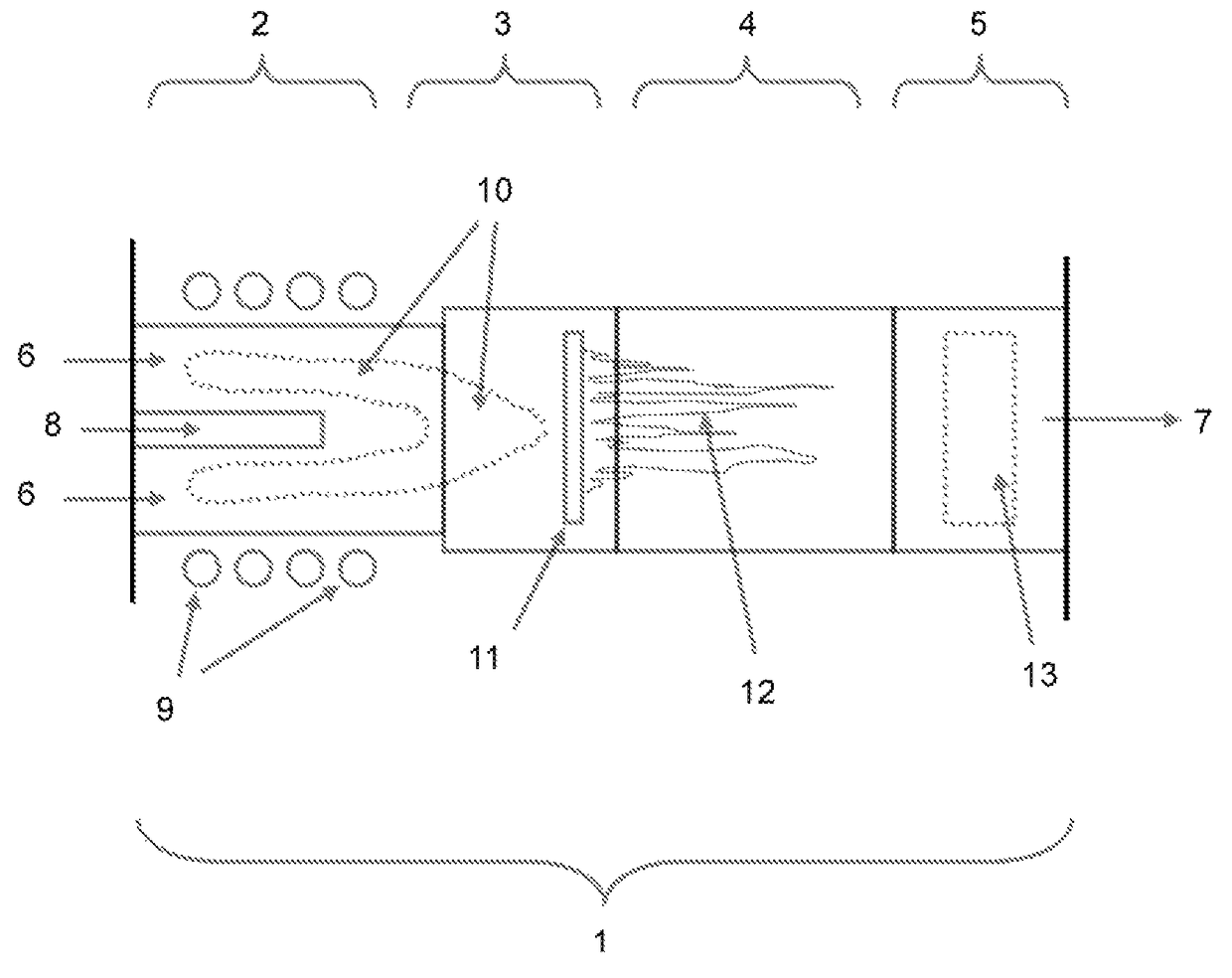
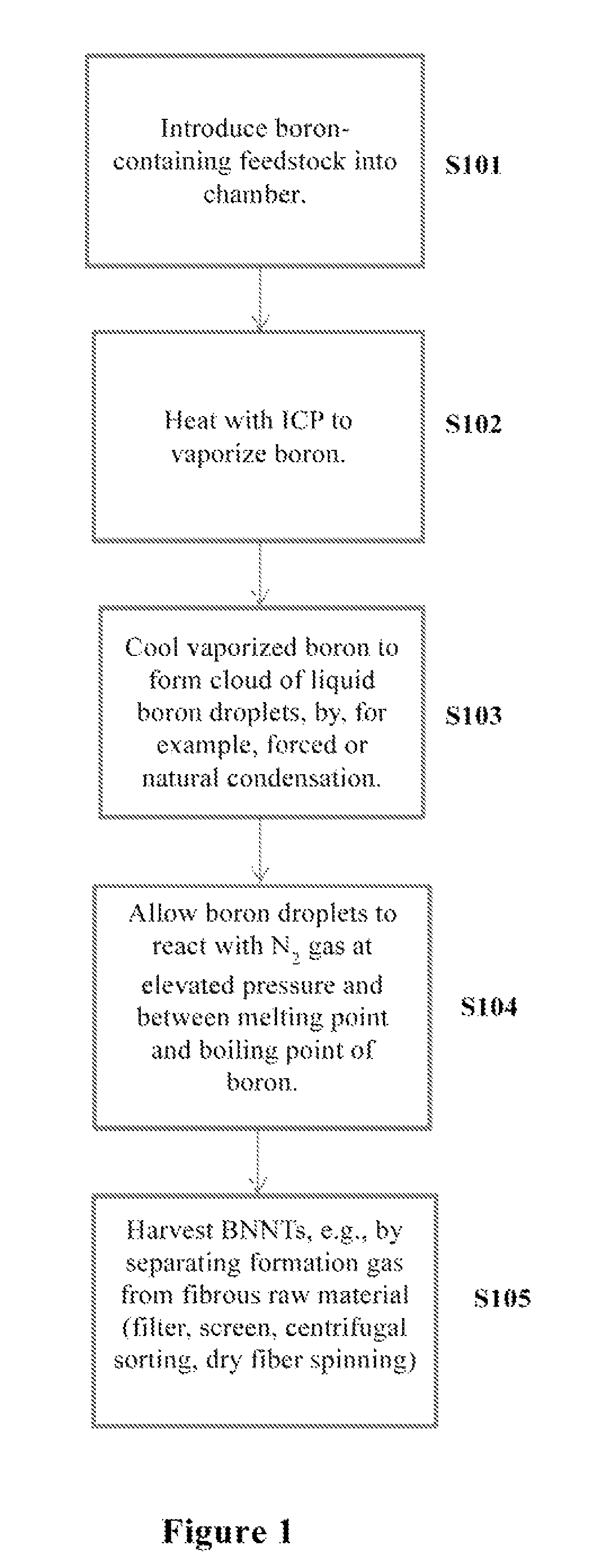
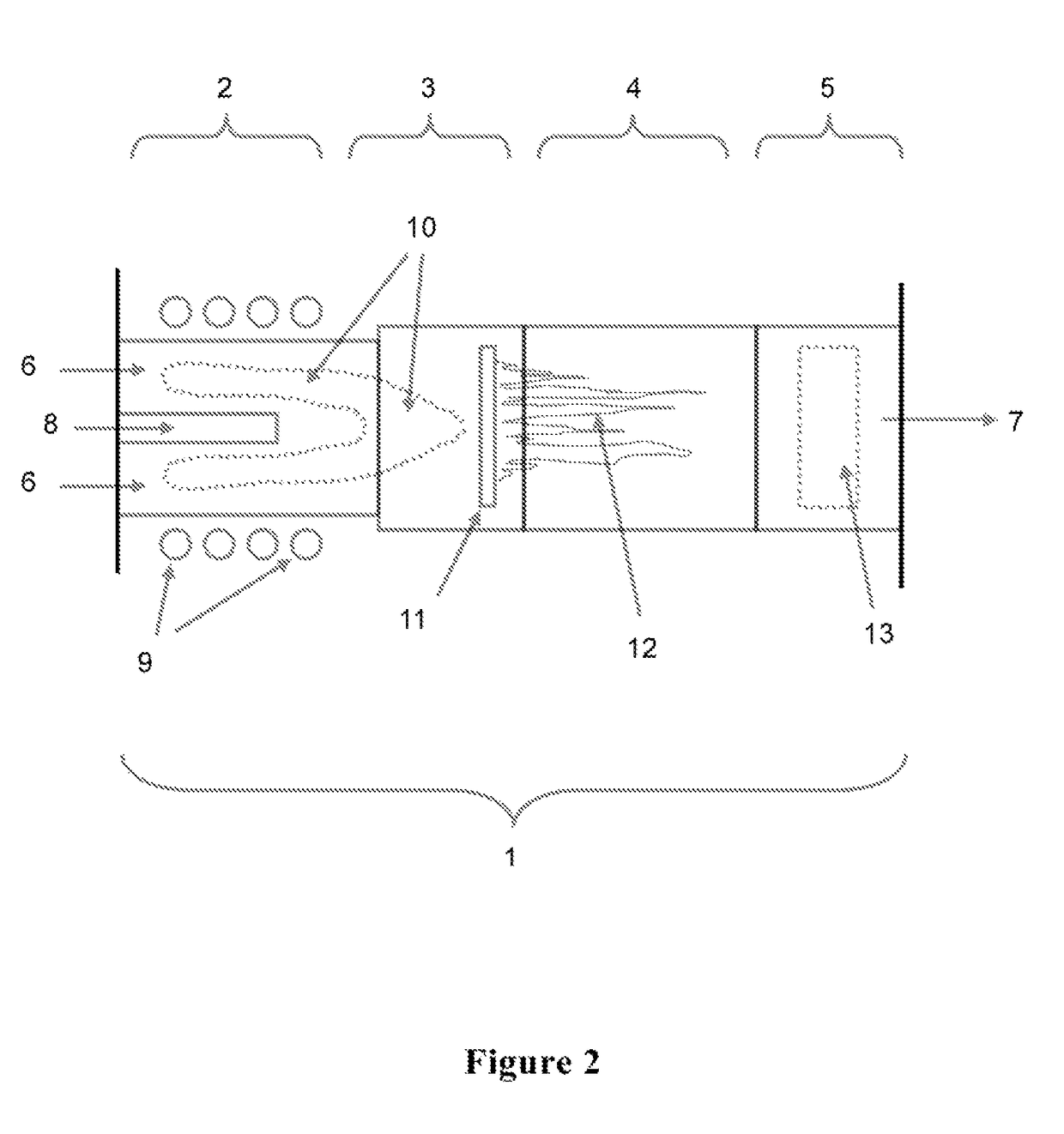
Induction-coupled plasma synthesis of boron nitride nanotubes
ActiveUS20150125374A1Few crystalline structure defectHigh yieldNitrogen compoundsHigh temperature liquid-gas reactionBoron nitrideNitrogen gas
Described herein are processes and apparatus for the large-scale synthesis of boron nitride nanotubes (BNNTs) by induction-coupled plasma (ICP). A boron-containing feedstock may be heated by ICP in the presence of nitrogen gas at an elevated pressure, to form vaporized boron. The vaporized boron may be cooled to form boron droplets, such as nanodroplets. Cooling may take place using a condenser, for example. BNNTs may then form downstream and can be harvested.
Owner:BNNT
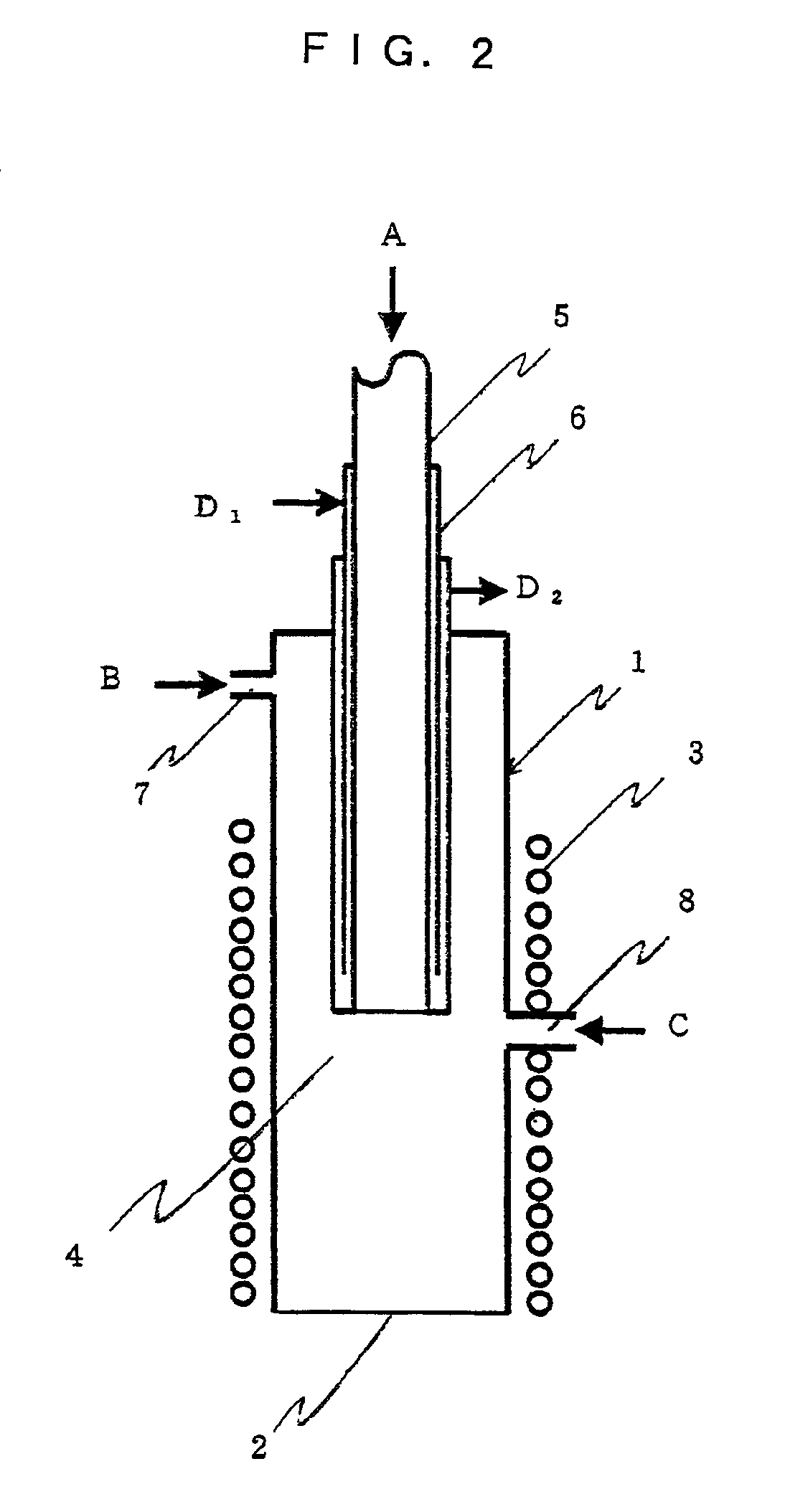
Polycrystalline silicon and process and apparatus for producing the same
InactiveUS6861144B2High stability and reproducibilityEfficiently obtainedSemiconductor/solid-state device manufacturingFrom frozen solutionsApparent densityFine grain
Owner:TOKUYAMA CORP
Molten metal reactor and method of forming hydrogen, carbon monoxide and carbon dioxide using the molten alkaline metal reactor
InactiveUS20110089377A1Extended stayCarbon monoxideHydrogen/synthetic gas productionHydrogenCrucible
A molten metal reactor for converting a carbon material and steam into a gas comprising hydrogen, carbon monoxide, and carbon dioxide is disclosed. The reactor includes an interior crucible having a portion contained within an exterior crucible. The interior crucible includes an inlet and an outlet; the outlet leads to the exterior crucible and may comprise a diffuser. The exterior crucible may contain a molten alkaline metal compound. Contained between the exterior crucible and the interior crucible is at least one baffle.
Owner:BATTELLE ENERGY ALLIANCE LLC
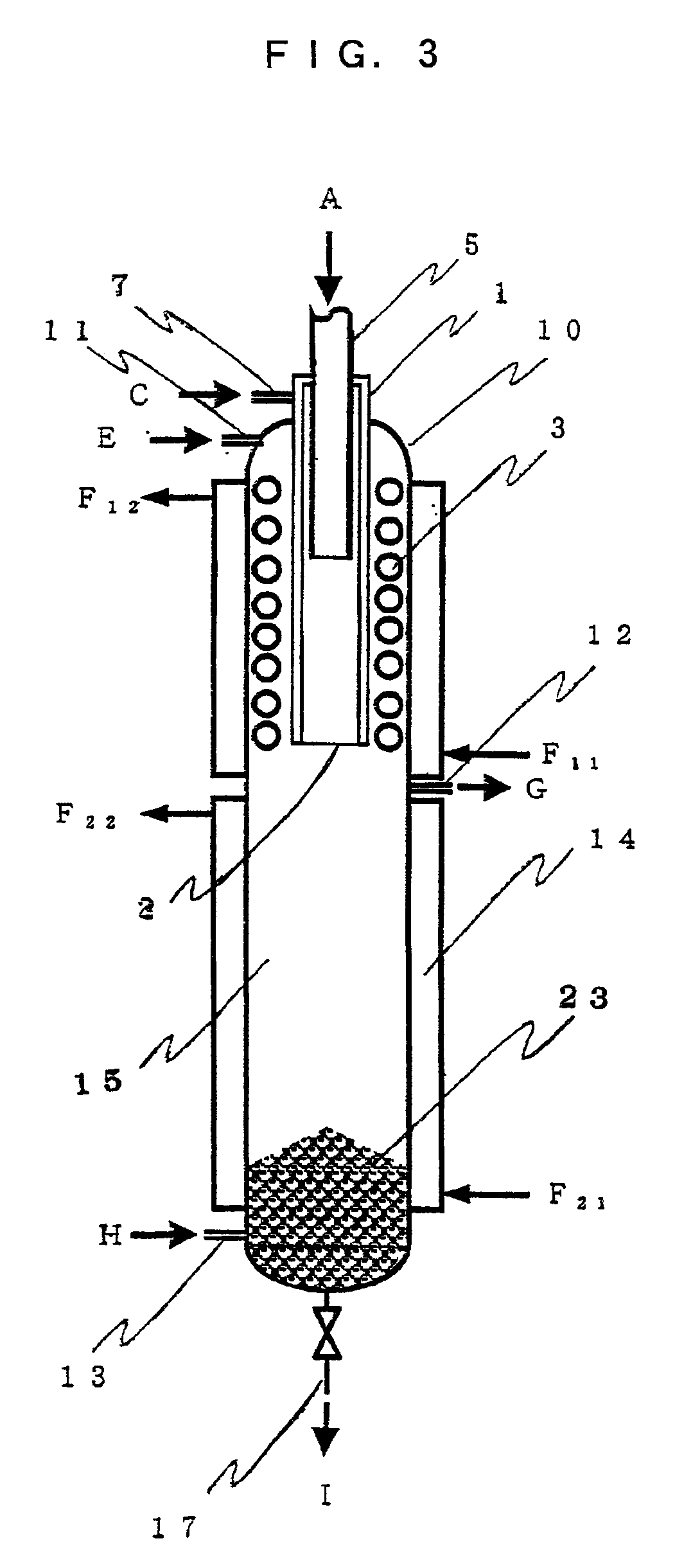
Production of hydrogen from water using a thermochemical copper-chlorine cycle
ActiveUS20100129287A1Heat produced can be efficientlyEffective recoveryCellsOxygen/ozone/oxide/hydroxideWater useDecomposition
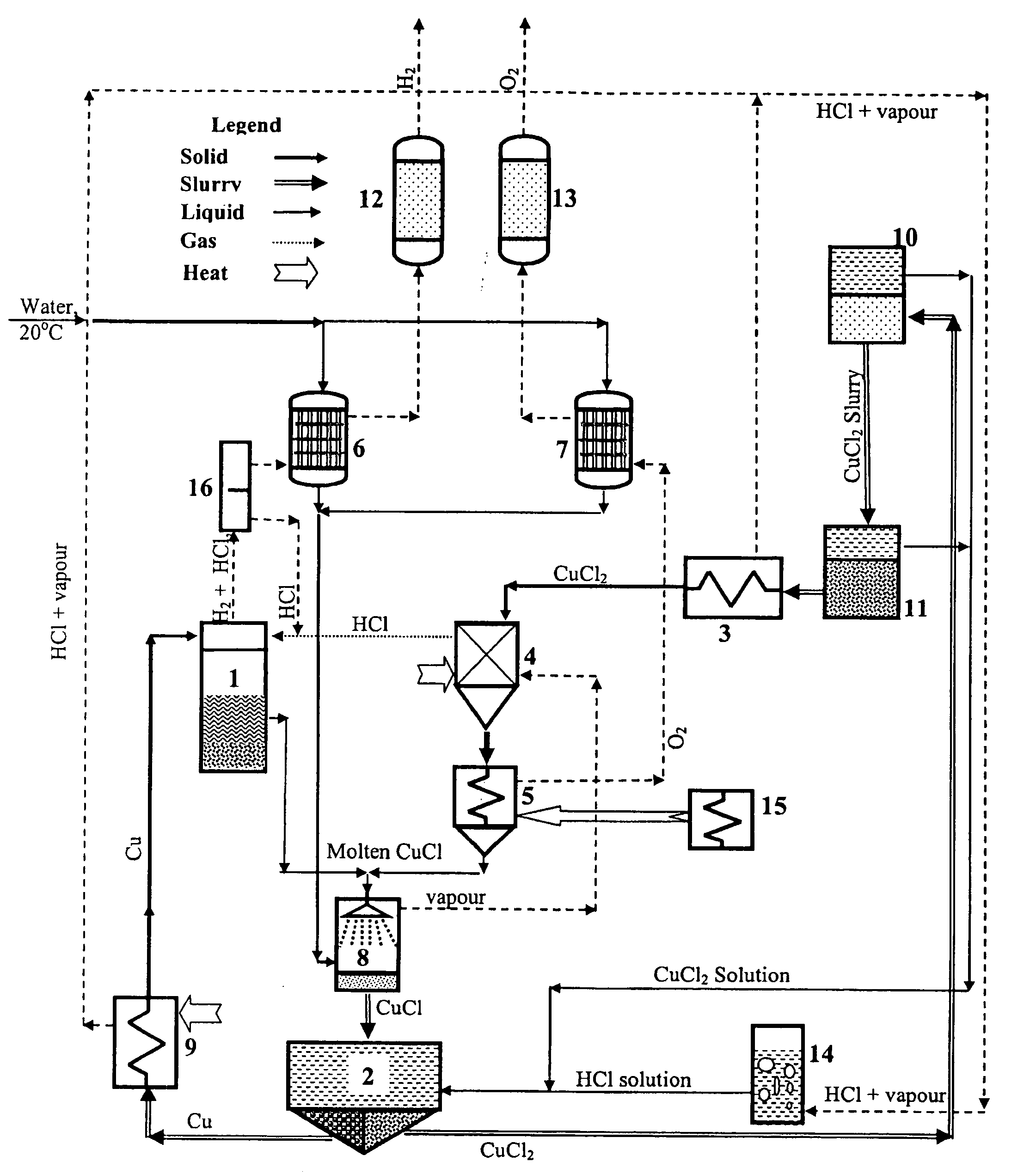
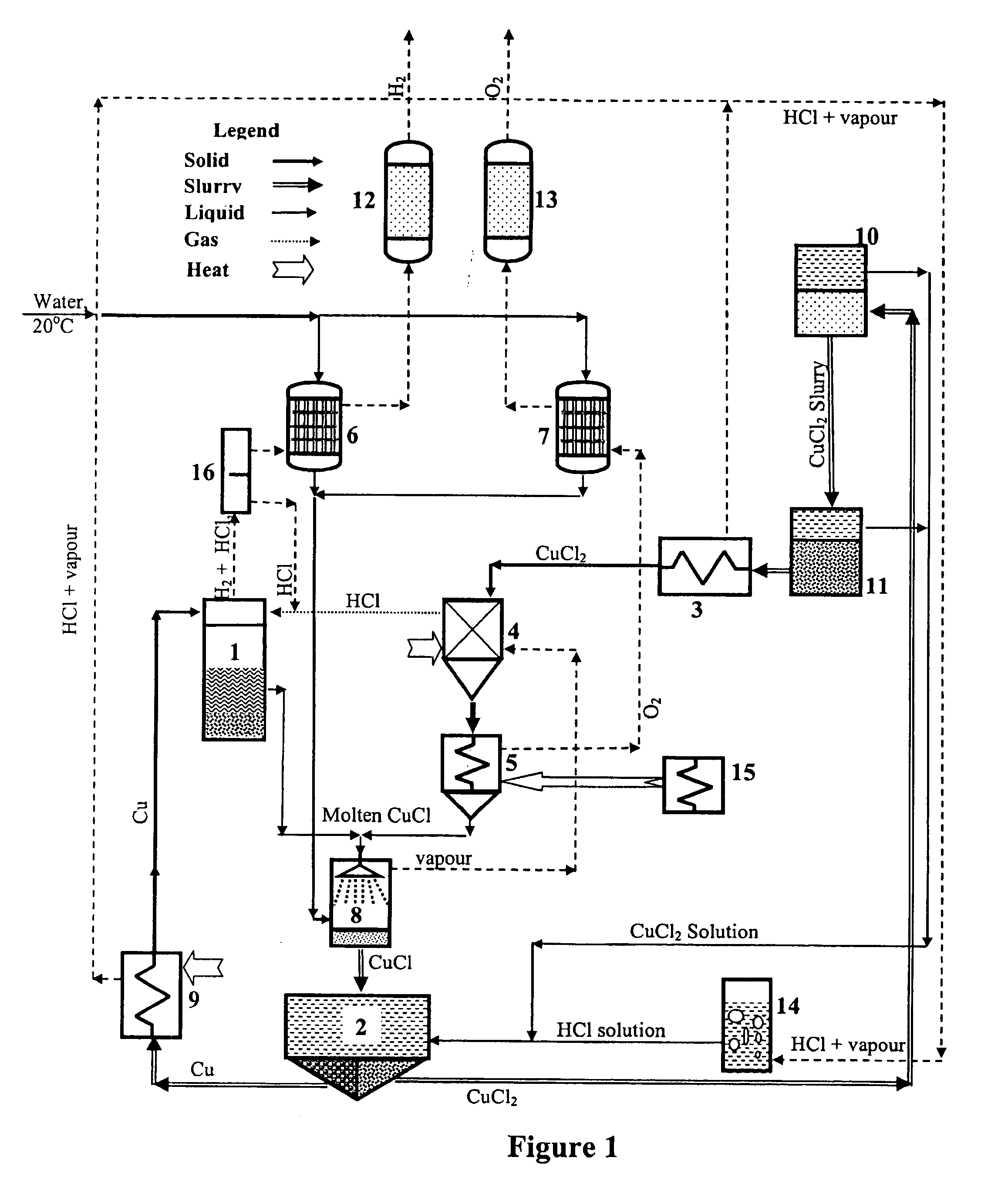
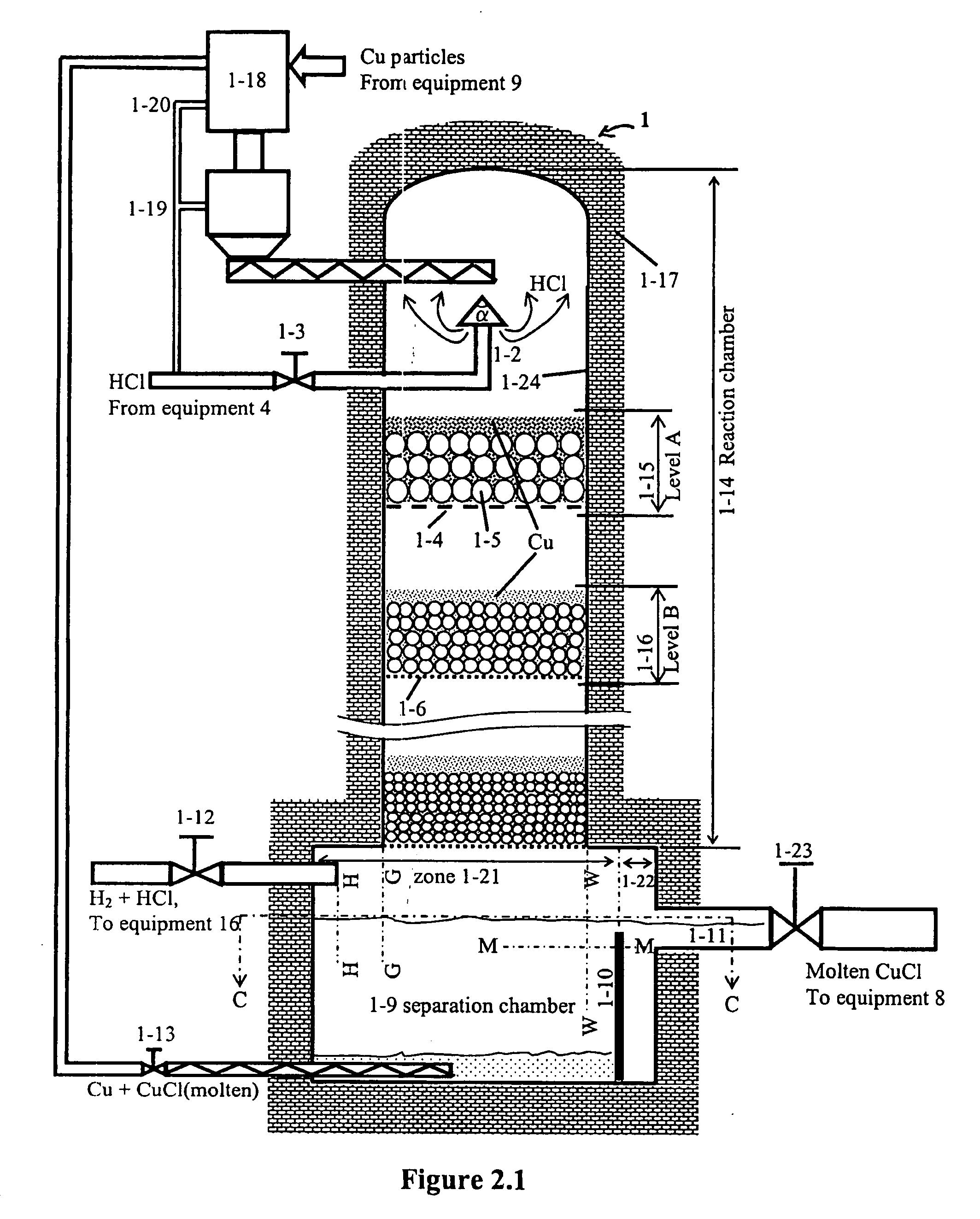
A system for producing hydrogen gas from water decomposition using a thermochemical CuCl cycle, the improvement comprising the use of an insulated hydrogen production reactor comprising a reaction chamber and a separation chamber; the reaction chamber having a hydrogen chloride gas inlet and a solid copper inlet; one or more levels provided in the reaction chamber, the number of which is dependant on production scale and pressure drop; each level comprising a perforated plate with associated filter media, the perforations of the plate and media being of decreasing size from top to bottom of the reaction chamber, and being sized to permit downward flow of the hydrogen gas and molten CuCl products, as well as the HCL gas reactant, and to prevent entrainment of solid copper in the molten CuCl; the separation chamber being located below the reaction chamber and being of greater cross section than the reaction chamber and comprising a first hydrogen removal and entrained copper removal zone and a second molten CuCl removal zone; removal of the reaction products being controlled so as to substantially decrease the amount of entrained copper in the molten CuCl; and the first zone having outlets for removal of hydrogen gas and entrained copper particles, with the second zone having an outlet for removal of molten CuCl.
Owner:UNIV OF ONTARIO INST OF TECH
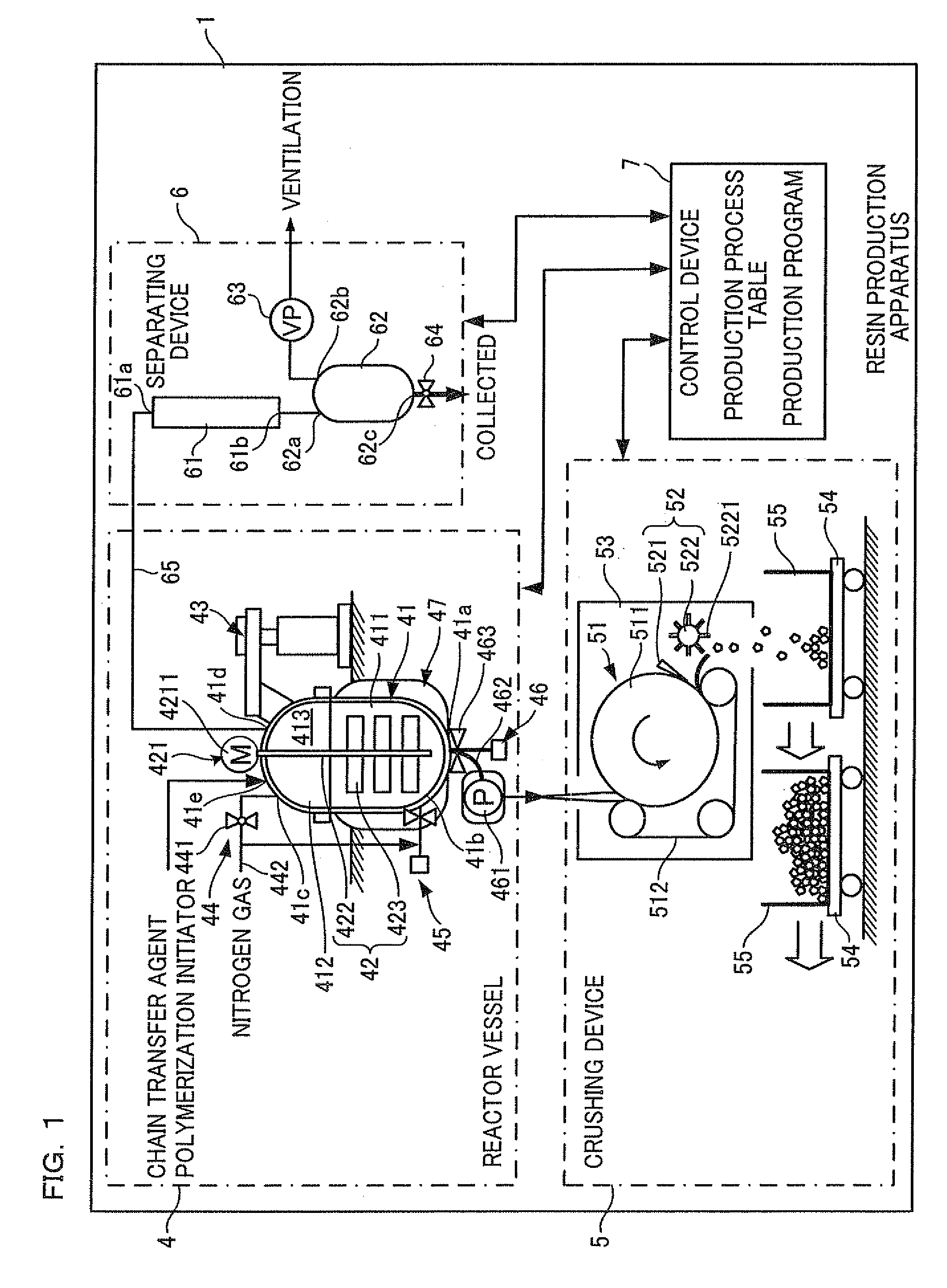
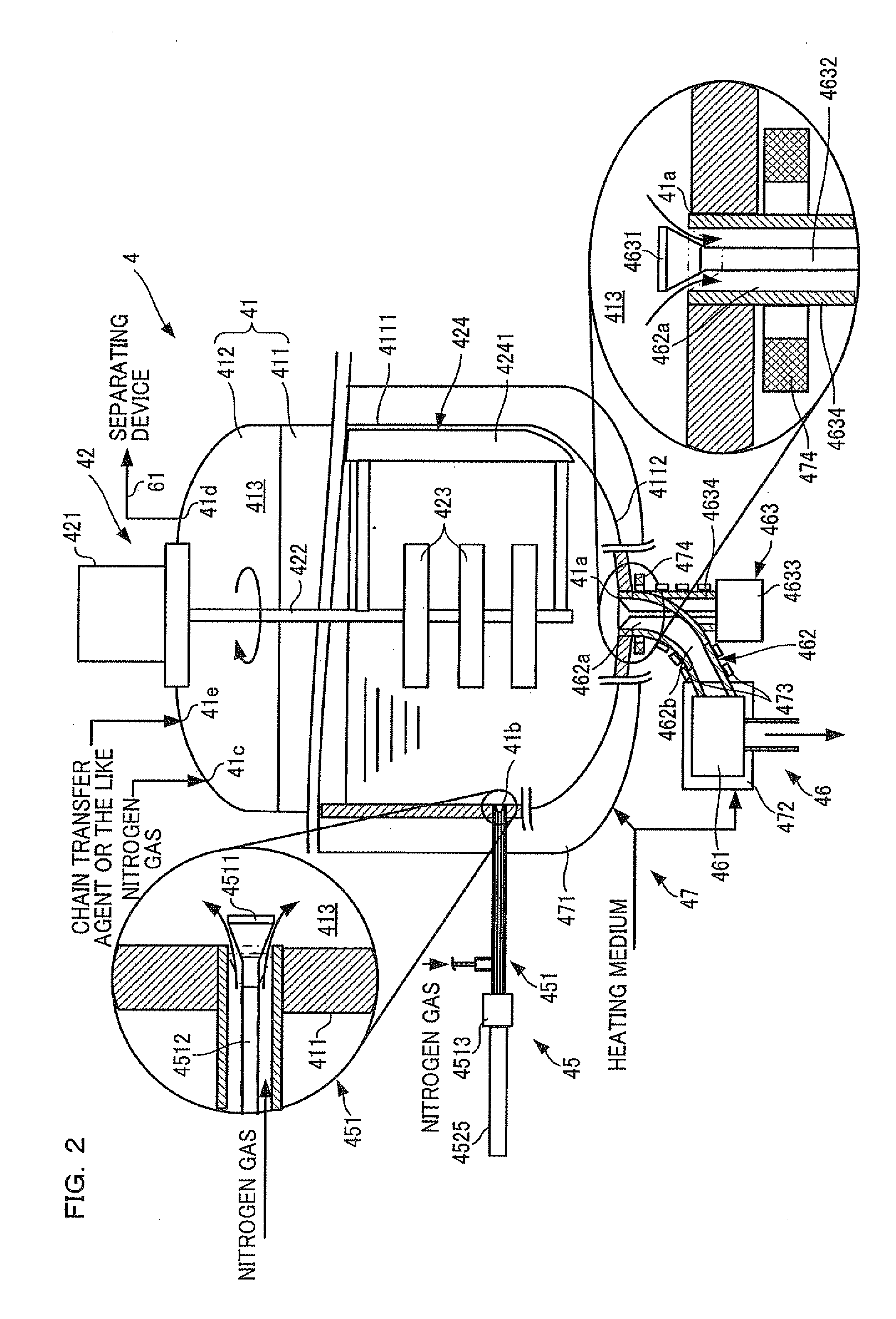
Resin production apparatus and resin production method
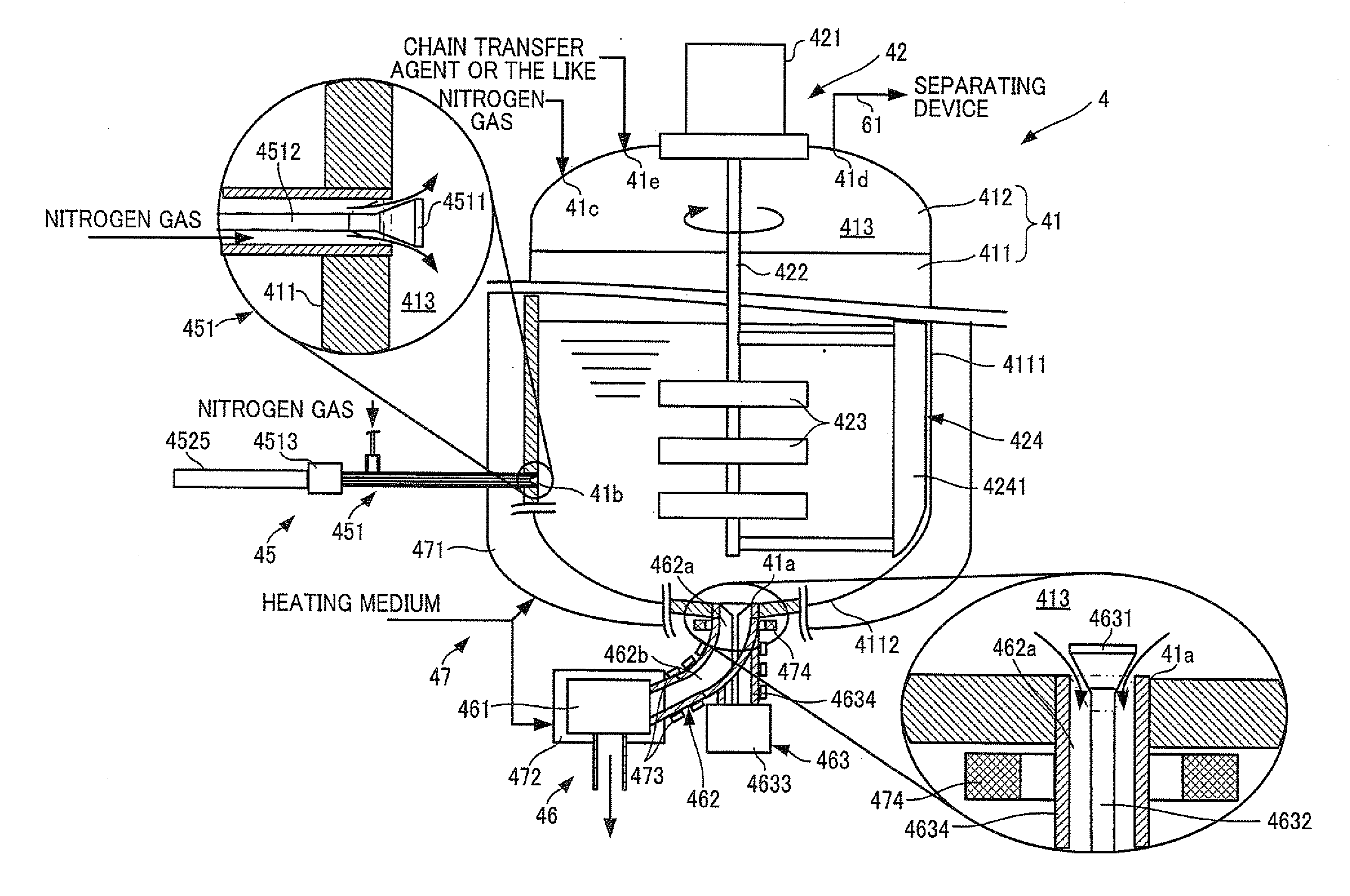
InactiveUS20110152564A1Reduces time and burdenLower the volumeProcess control/regulationOrganic chemistryMolten stateMolten salt
A resin production apparatus of the present invention includes: a reactor vessel having a vessel main body which polymerizes an ingredient to produce a thermoplastic synthetic resin which solidifies at room temperature and storing the synthetic resin in the molten state, an output mechanism disposed at a bottom part of the vessel main body, which outputs the synthetic resin in the molten state, and a temperature adjustment mechanism which adjusts temperatures of the vessel main body and the output mechanism so as to maintain the molten state of the synthetic resin; a cooling mechanism arranged below the reactor vessel, which continuously cools and solidifies the synthetic resin output from the output mechanism; and a crushing mechanism which crushes the synthetic resin fed out from the cooling mechanism.
Owner:NITTO DENKO CORP
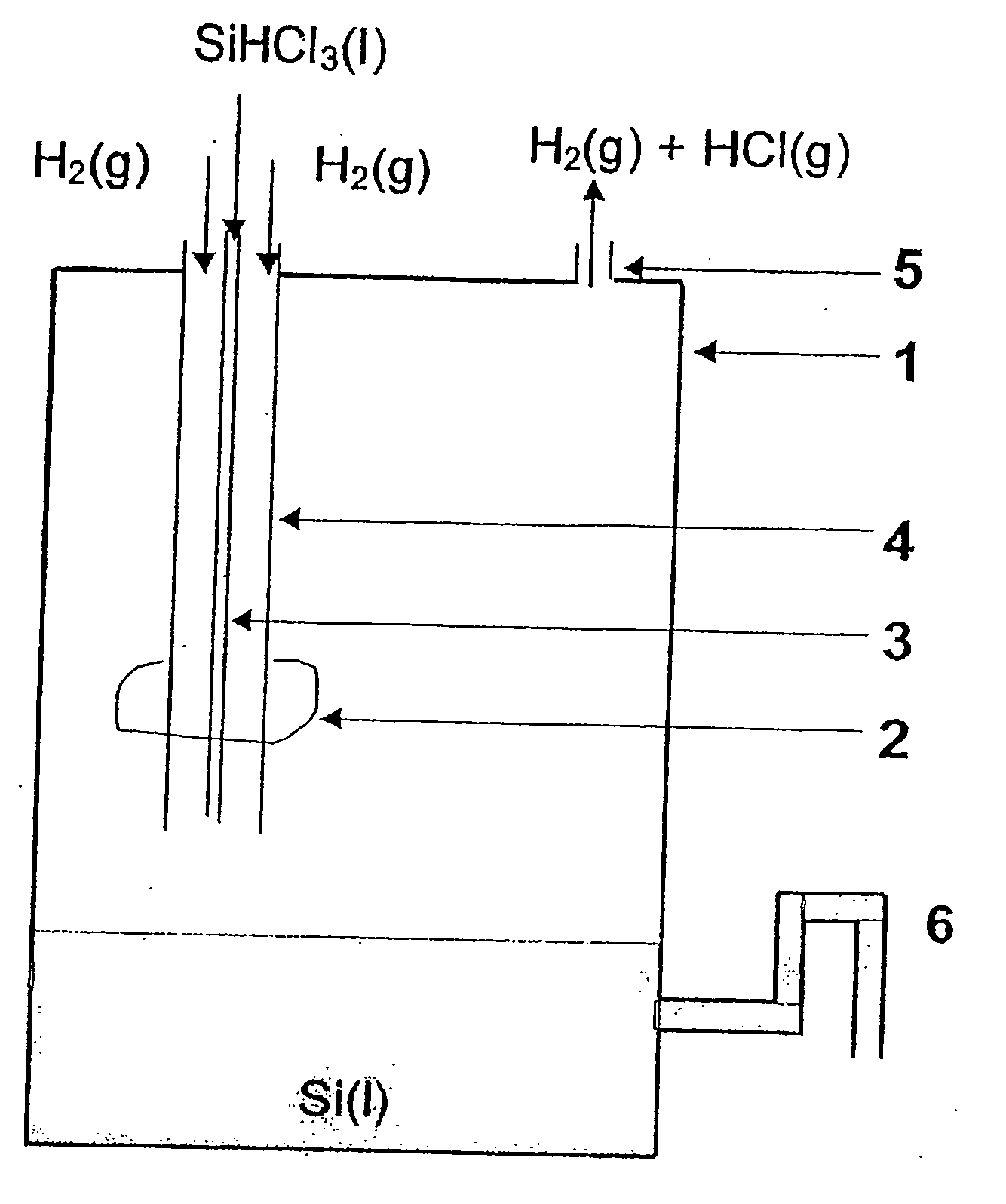
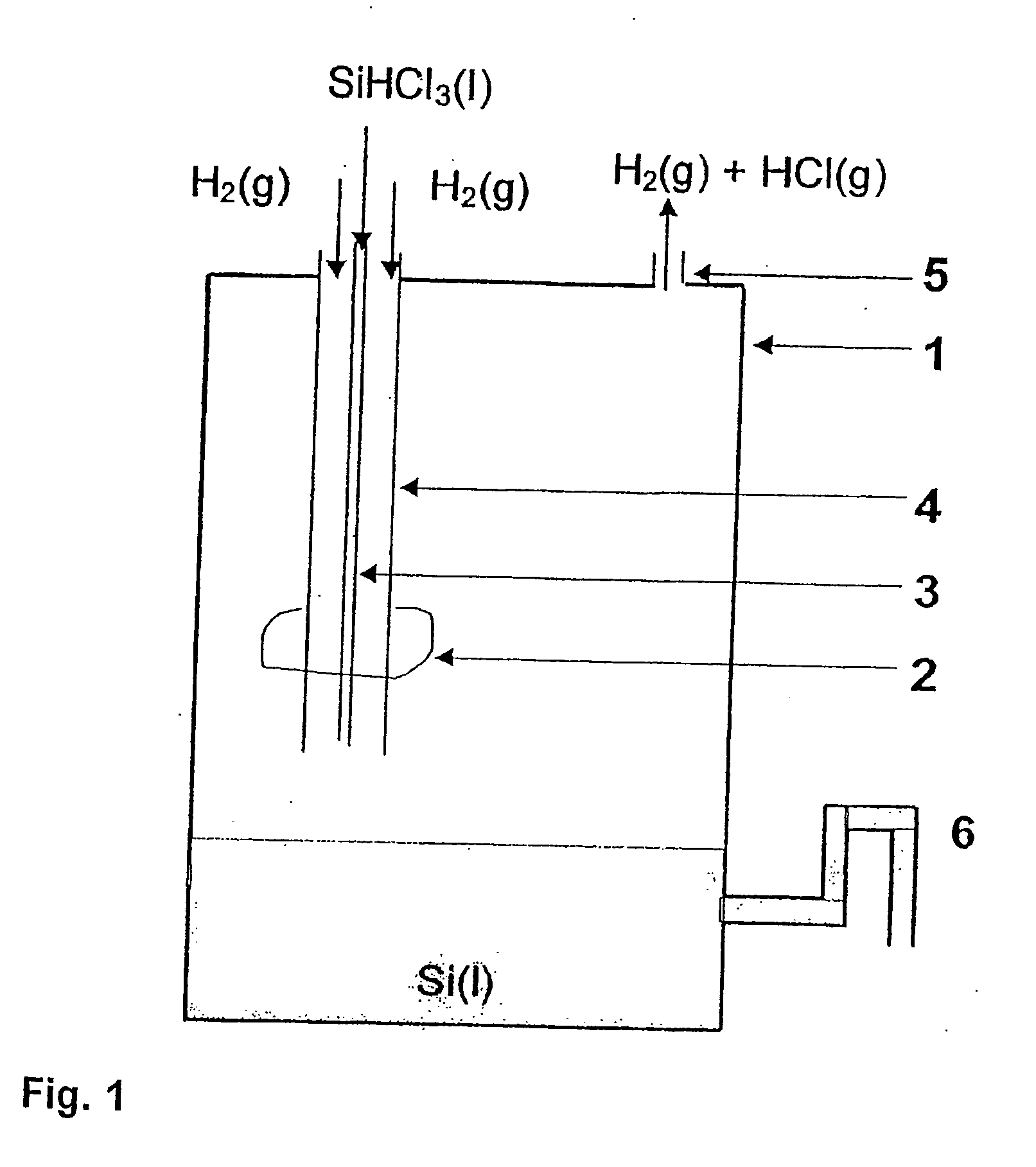
Production of high grade silicon, reactor, particle recapture tower and use of the aforementioned
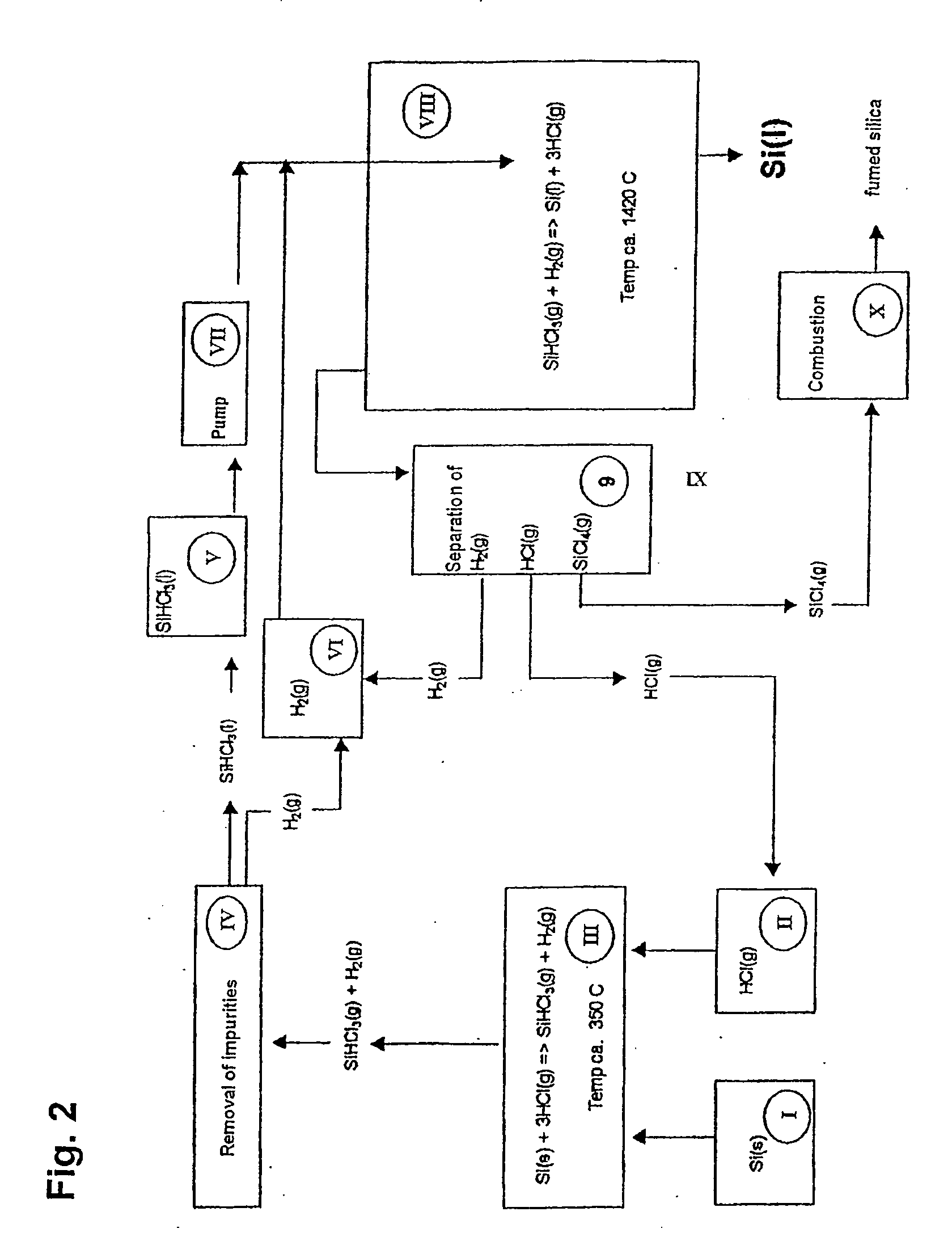
Solar Grade Silicon is produced by decomposition of a silicon precursor, preferably trichlorosilane, in the presence of an excess of hydrogen gas, where the reactant are introduced in a reaction chamber whose lower portion is held at a temperature above the melting point of silicon and whose upper portion is held at ambient temperatures. The method is distinguished by the introduction of trichlorosilane through a feed pipe which is arranged coaxially inside an outer pipe for introducing hydrogen gas that functions as a cooling medium for the introduced fluid trichlorosilane. The silicon formed is collected in the lower portion of the reactor and removed through an outlet. Excess hydrogen and hydrogen chloride is withdrawn through an outlet and can, after purification, be used as reactants in the essentially closed system for the production of pure silicon from low grade silicon. Silicon particles in the off gases can be separated, melted and recycled using a particle recapture tower.
Owner:SOLAR GRADE SILICON
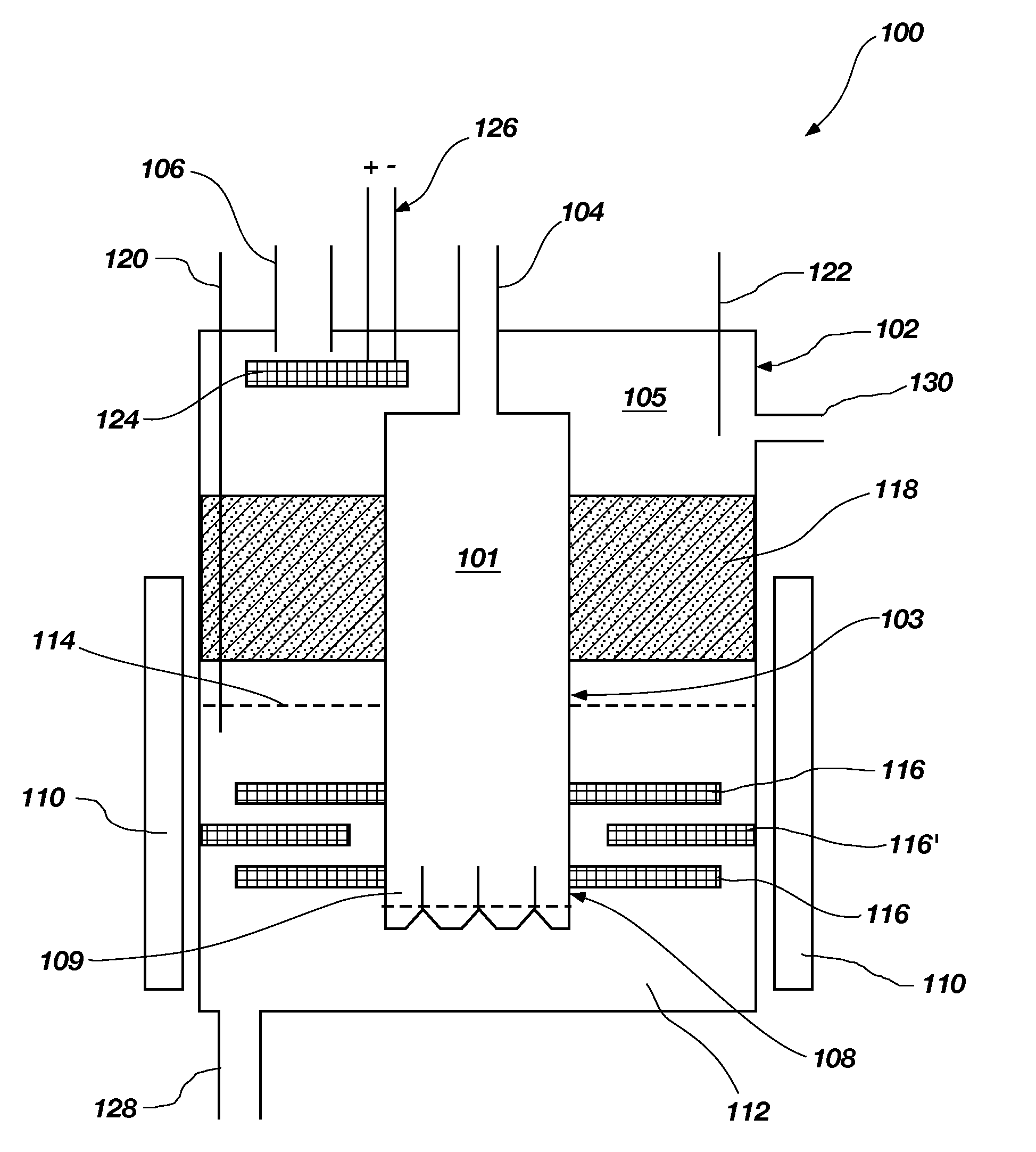
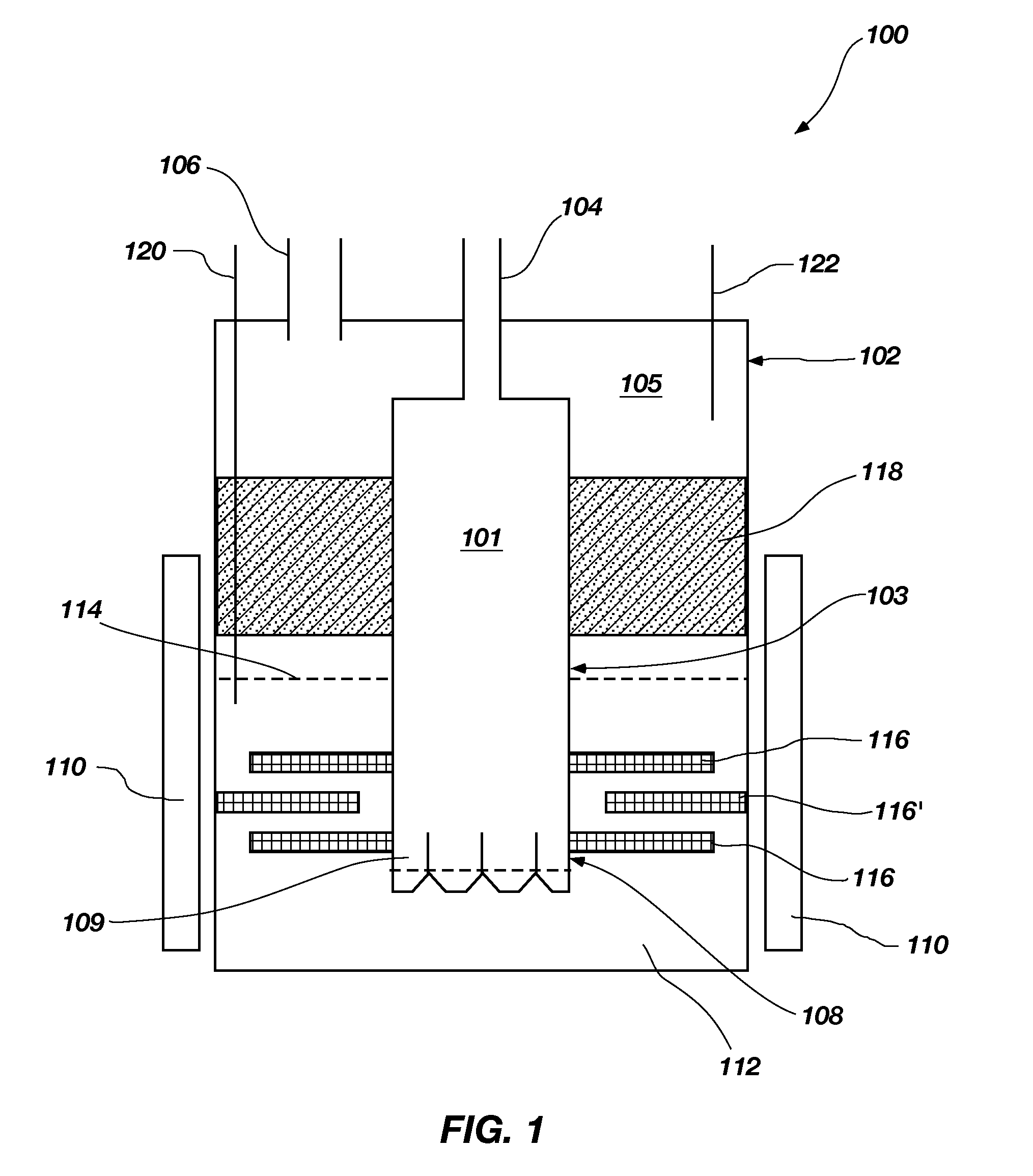
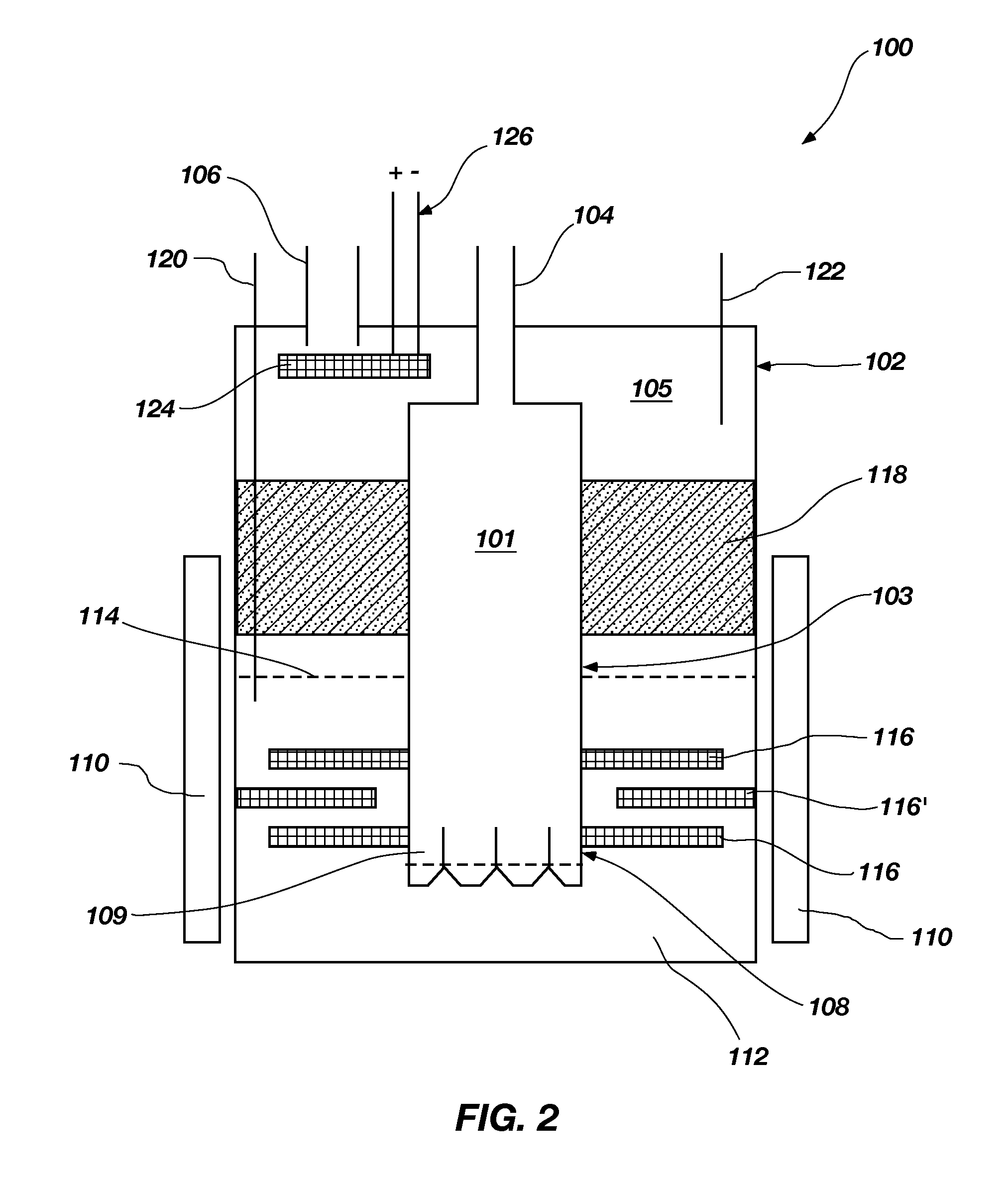
Systems and methods for maintaining chemistry in molten salt systems
InactiveUS20200122109A1Reduce solubilityFacilitate chemical reactionsElectrolysis componentsLiquid-gas reaction as foam/aerosol/bubblesChemical controlPhysical chemistry
Methods and systems for removing impurities from a molten salt stream are provided. A molten salt stream is provided that comprises a mixture of compounds selected from the group consisting of LiF, BeF2, and NaF, and ZrF4. The molten salt stream is flowed through a loop that may contain a precipitation filter, electrochemical potential, and / or a sparger, which thereby remove impurities in the molten salt stream. Various physical methods and apparatus are used to control the ability to remove impurities from the molten salt stream based on temperature, solubility, and general chemistry control.
Owner:KAIROS POWER LLC
Molten salt treatment system and process
InactiveCN102256693AUsing liquid separation agentHigh temperature liquid-gas reactionEngineeringProduct gas
Owner:TALE & LYLE TECH LTD
Molten metal reactor utilizing molten metal flow for feed material and reaction product entrapment
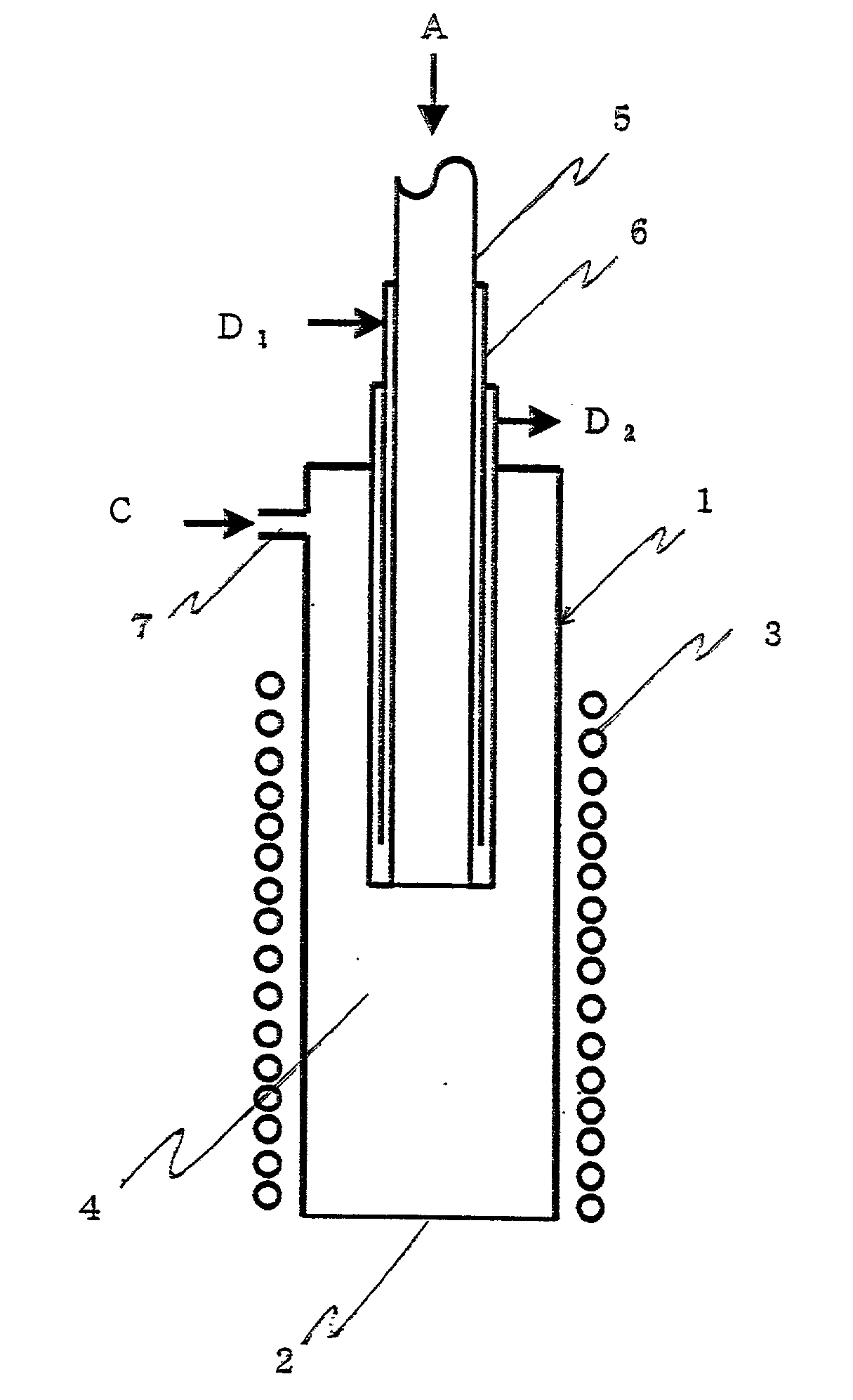
InactiveUS7449156B2Desired contactSpeed up the flowInorganic chemistryCremation furnacesPhysical chemistryMaterials science
A molten metal reactor (10) quickly entrains a feed material in the molten reactant metal (16) and provides the necessary contact between the molten reactant metal and the feed material to effect the desired chemical reduction of the feed material. The reactor (10) includes a unique feed structure (24) adapted to quickly entrain the feed material into the molten reactant metal (16) and then transfer the molten reactant metal, feed material, and initial reaction products into a treatment chamber (12). A majority of the desired reactions occur in the treatment chamber (12). Reaction products and unspent reactant metal are directed from the treatment chamber (12) to an output chamber (14) where reaction products are removed from the reactor. Unspent reactant metal (16) is then transferred to a heating chamber (15) where it is reheated for recycling through the system.
Owner:CLEAN TECH INT
Integrated Process For Steam Cracking
ActiveUS20120006723A1Thermal non-catalytic crackingTreatment with plural serial cracking stages onlyVisbreakerBoiling point
This invention relates to a process and system for cracking hydrocarbon feedstock containing vacuum resid comprising: (a) subjecting a vacuum resid to a first thermal conversion in a thermal conversion reactor (such as delayed coker, fluid coker, Flexicoker™, visbreaker and catalytic hydrovisbreaker) where at least 30 wt % of the vacuum resid is converted to material boiling below 1050° F. (566° C.); (b) introducing said thermally converted resid to a vapor / liquid separator, said separator being integrated into a steam cracking furnace, to form a vapor phase and liquid phase; (c) passing said vapor phase to the radiant furnace in said steam cracking furnace; and (d) recovering at least 30 wt % olefins from the material exiting the radiant furnace (based upon the weight of the total hydrocarbon material exiting the radiant furnace).
Owner:EXXONMOBIL CHEM PAT INC
Induction-coupled plasma synthesis of boron nitride nanotubes
ActiveUS9776865B2Long and flexibleCrystal structure defectNitrogen compoundsHigh temperature liquid-gas reactionBoron containingBoron nitride
Owner:BNNT
Reaction vessel and process for its use
InactiveUS20100015037A1Severe corrosionExtended stayGaseous chemical processesSulfur-dioxide/sulfurous-acidHydrogenChemistry
The invention relates to a reaction vessel in which hydrogen sulphide is prepared from sulphur and hydrogen, wherein the reaction vessel consists partly or entirely of a material which is resistant to the reaction mixture, its compounds or elements and retains its resistance even at high temperatures.
Owner:EVONIK DEGUSSA GMBH
Integrated process for steam cracking
ActiveUS8399729B2Thermal non-catalytic crackingTreatment with plural serial cracking stages onlyVisbreakerBoiling point
This invention relates to a process and system for cracking hydrocarbon feedstock containing vacuum resid comprising: (a) subjecting a vacuum resid to a first thermal conversion in a thermal conversion reactor (such as delayed coker, fluid coker, Flexicoker™, visbreaker and catalytic hydrovisbreaker) where at least 30 wt % of the vacuum resid is converted to material boiling below 1050° F. (566° C.); (b) introducing said thermally converted resid to a vapor / liquid separator, said separator being integrated into a steam cracking furnace, to form a vapor phase and liquid phase; (c) passing said vapor phase to the radiant furnace in said steam cracking furnace; and (d) recovering at least 30 wt % olefins from the material exiting the radiant furnace (based upon the weight of the total hydrocarbon material exiting the radiant furnace).
Owner:EXXONMOBIL CHEM PAT INC
Reaction vessel and process for its use
InactiveUS7833508B2Severe corrosionExtended stayGaseous chemical processesSulfur-dioxide/sulfurous-acidHydrogenChemistry
The invention relates to a reaction vessel in which hydrogen sulphide is prepared from sulphur and hydrogen, wherein the reaction vessel consists partly or entirely of a material which is resistant to the reaction mixture, its compounds or elements and retains its resistance even at high temperatures.
Owner:EVONIK DEGUSSA GMBH
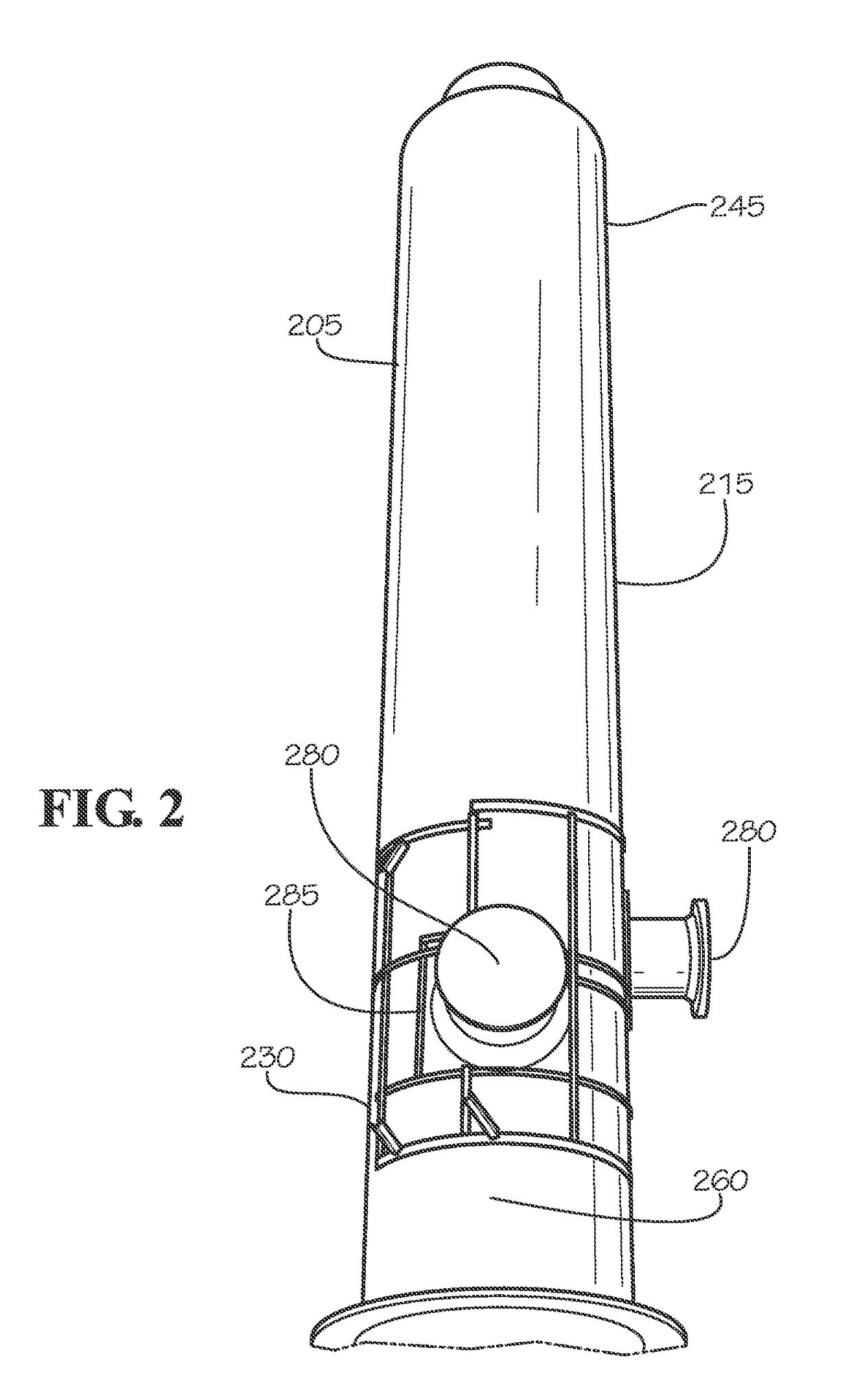
Molten salt rolling bubble column, reactors utilizing same and related methods
InactiveUS20130020232A1Hydrogen/synthetic gas productionChemical/physical/physico-chemical processesChemical reactionCrucible
Reactors for carrying out a chemical reaction, as well as related components, systems and methods are provided. In accordance with one embodiment, a reactor is provided that includes a furnace and a crucible positioned for heating by the furnace. The crucible may contain a molten salt bath. A downtube is disposed at least partially within the interior crucible along an axis. The downtube includes a conduit having a first end in communication with a carbon source and an outlet at a second end of the conduit for introducing the carbon material into the crucible. At least one opening is formed in the conduit between the first end and the second end to enable circulation of reaction components contained within the crucible through the conduit. An oxidizing material may be introduced through a bottom portion of the crucible in the form of gas bubbles to react with the other materials.
Owner:BATTELLE ENERGY ALLIANCE LLC
High pressure method for producing pure melamine in a vertical synthesis reactor
The invention relates to a high pressure method for producing pure melamine by pyrolyzing urea in a vertical synthesis reactor. The invention is characterized in that the synthesis reactor has three stages vertically arranged above one other, whereby: a) in the first i.e. uppermost stage, the smaller portion of the total amount of urea is introduced into the central tube of a first tank reactor whereby forming a first melamine-containing reaction medium; b) in the second i.e. middle stage, the first melamine-containing reaction medium as well as the larger portion of the total amount of urea is introduced into the central tube of a second tank reactor whereby forming a second melamine-containing reaction medium, after which; c) in the third i.e. lowermost stage, the second melamine-containing reaction medium is introduced into a vertical tubular flow reactor whereby forming a raw melamine melt that is subsequently processed in any manner whereby obtaining pure melamine. This makes it possible to achieve a more uniform conversion of urea, a more mild and corrosion-reducing supply of reaction heat, and for the reaction to be optimally carried out as well as to achieve a complete reaction of the urea in the melamine synthesis reactor. In comparison to other melamine methods, the invention provides a more compact, economical and efficient synthesis of melamine.
Owner:AMI AGROLINZ MELAMINE INT
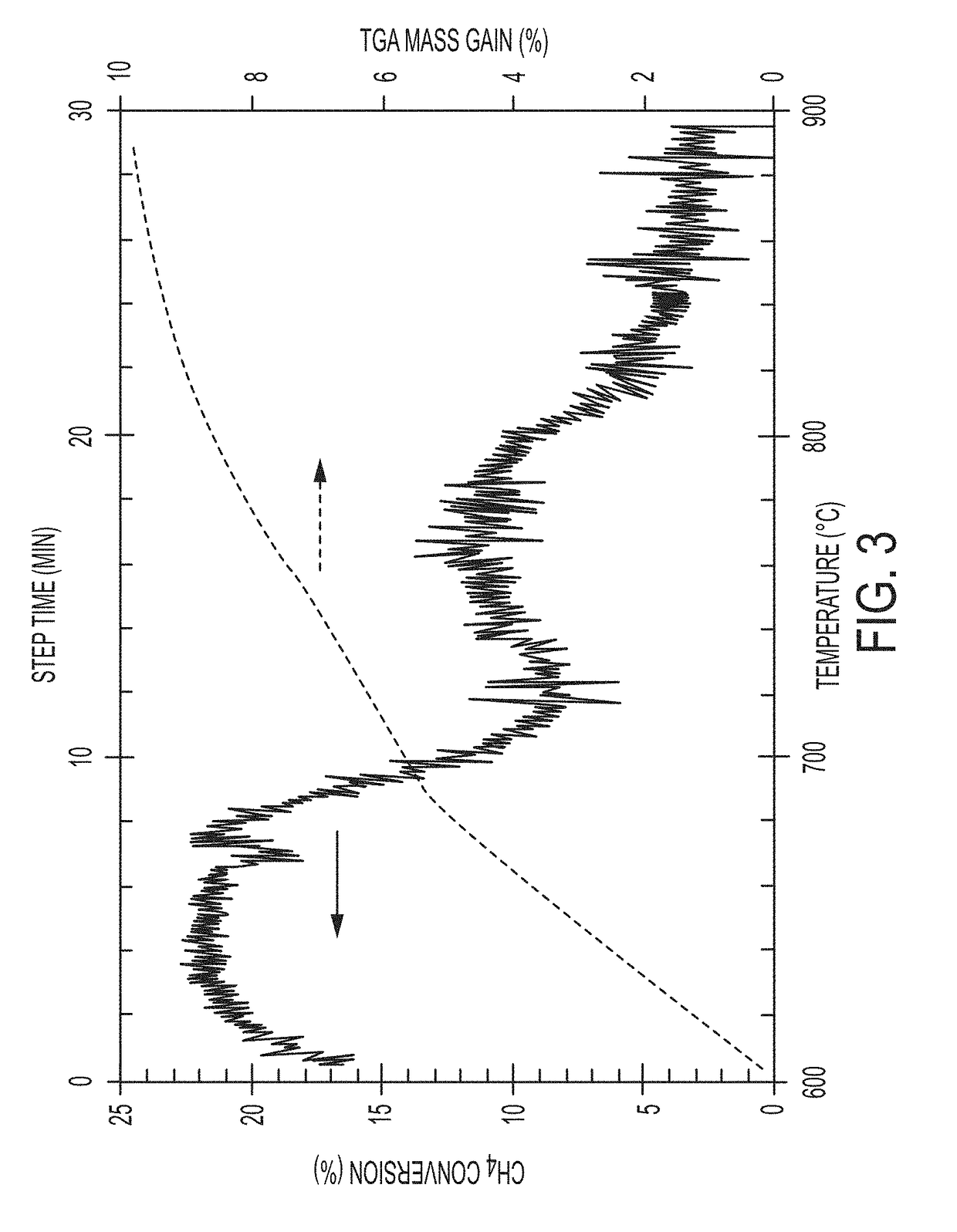
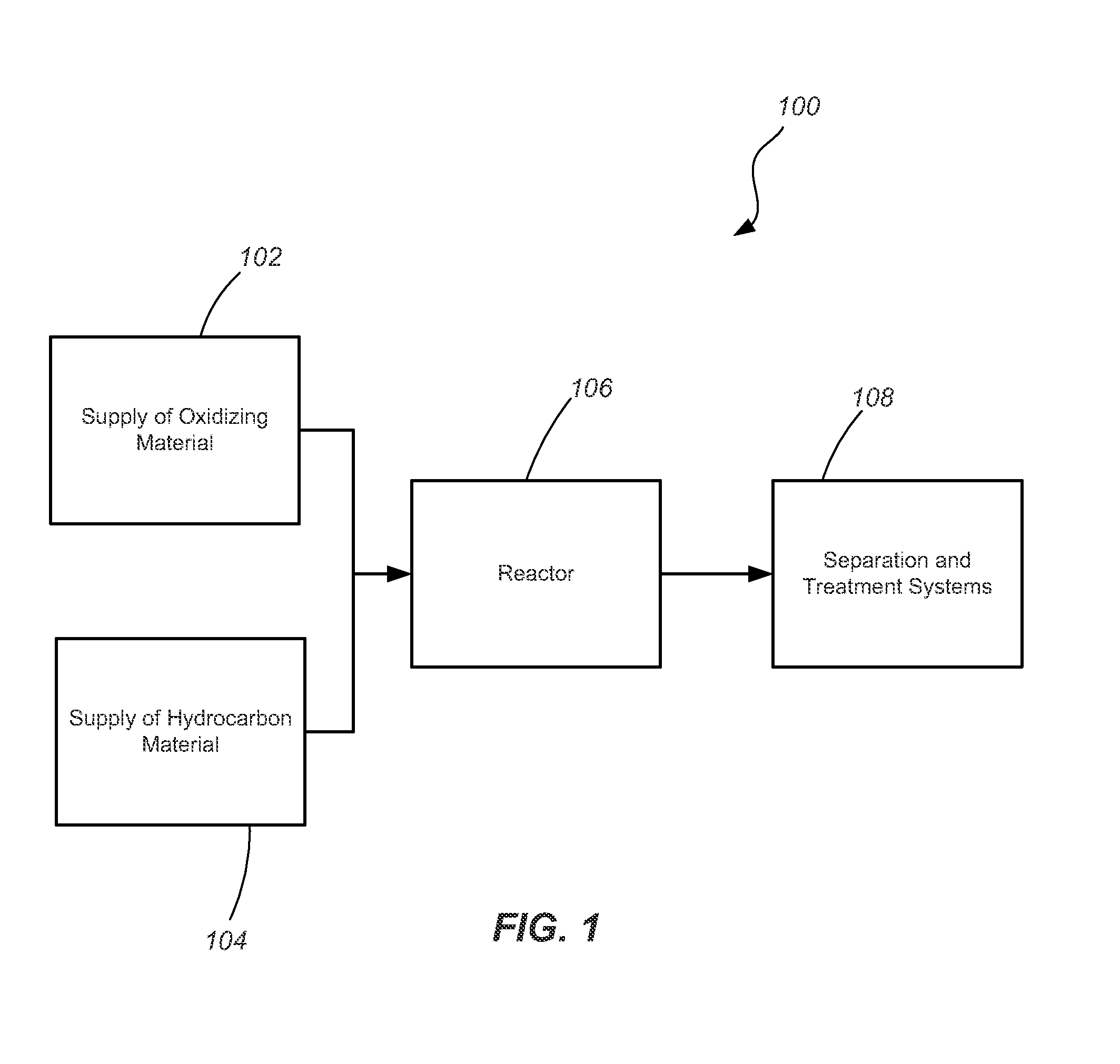
Simultaneous reaction and separation of chemicals
The reaction rate of hydrocarbon pyrolysis can be increased to produce solid carbon and hydrogen by the use of molten materials which have catalytic functionality to increase the rate of reaction andphysical properties that facilitate the formation and contamination-free separation of the solid carbon. Processes, materials, reactor configurations, and conditions are disclosed whereby methane andother hydrocarbons can be decomposed at high reaction rates into hydrogen gas and carbon products without any carbon oxides in a single reaction step. The process also makes use of specific propertiesof selected materials with unique solubilities and / or wettability of products into (and / or by) the molten phase to facilitate generation of purified products and increased conversion in more generalreactions.
Owner:RGT UNIV OF CALIFORNIA
Apparatus for producing metal oxide
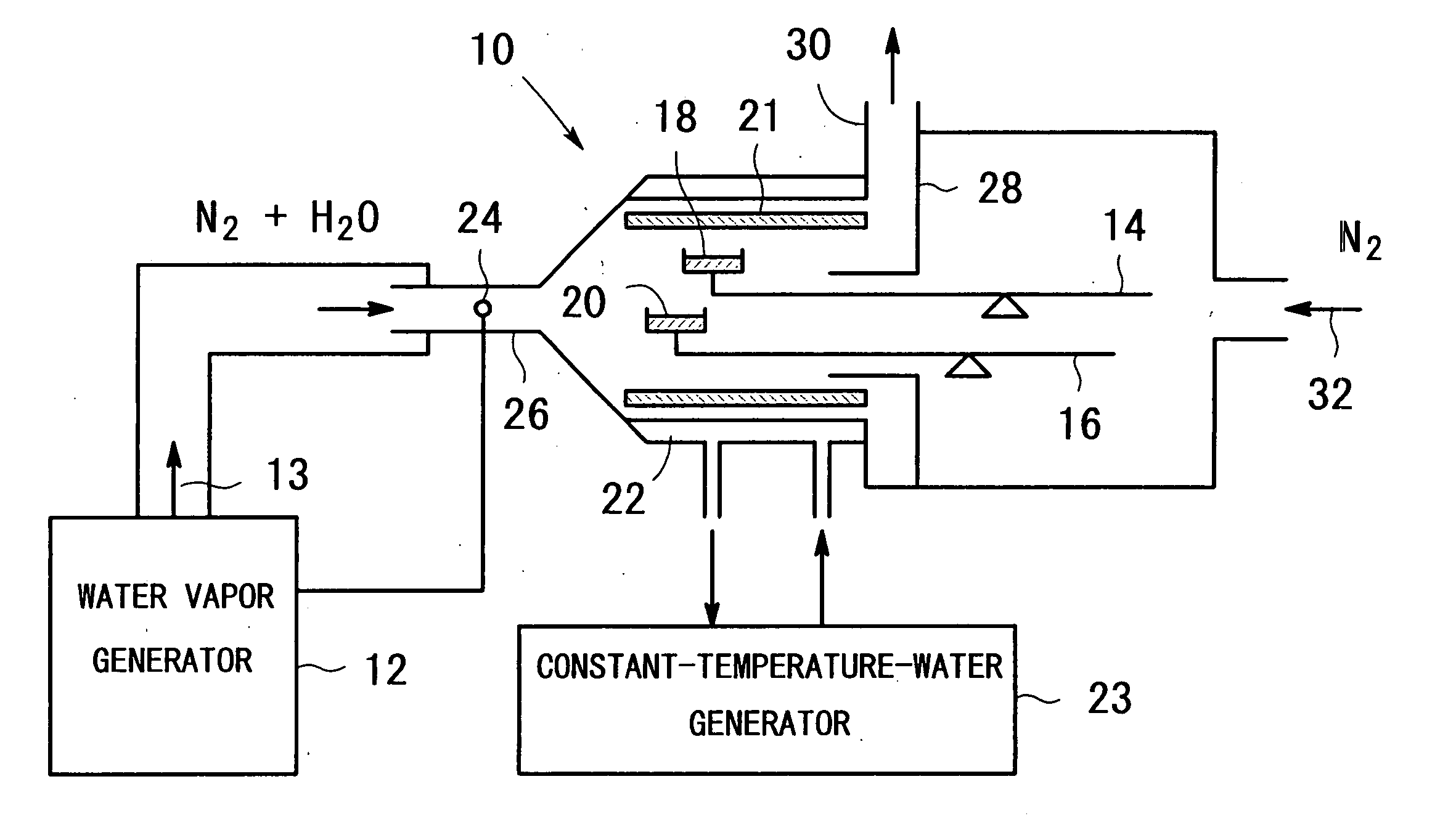
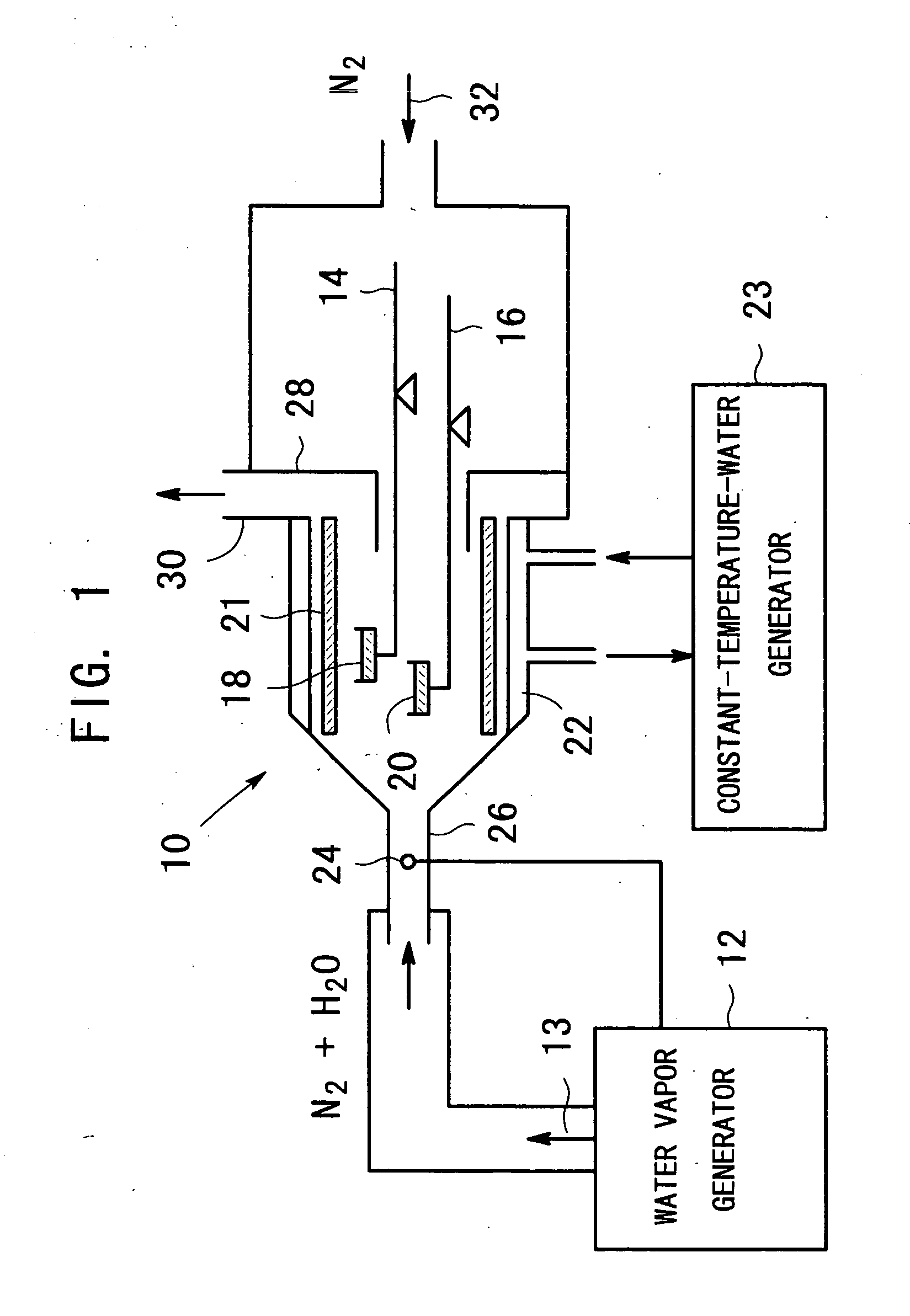
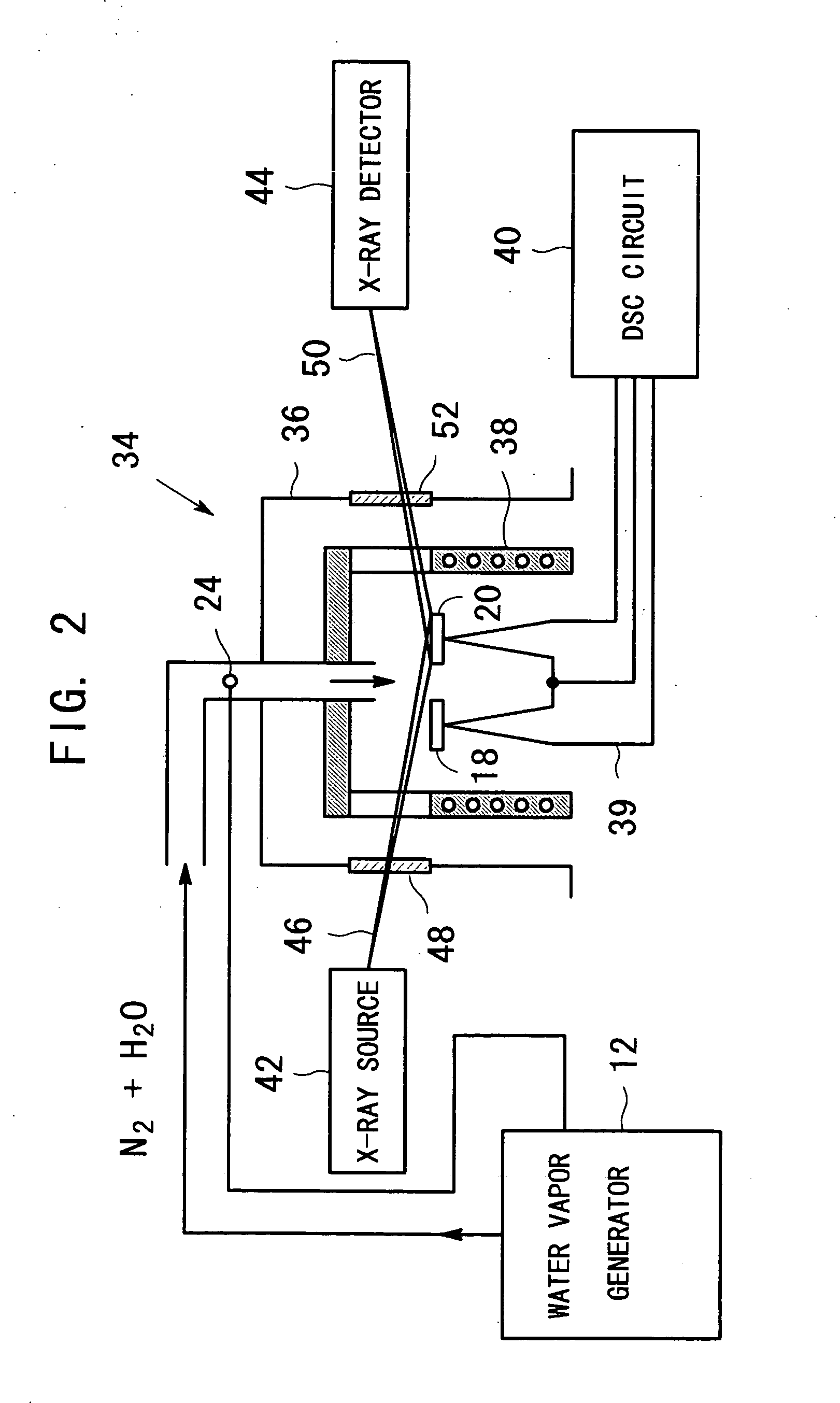
InactiveUS20050098112A1Avoid condensationIncrease valueOxide/hydroxide preparationZinc oxides/hydroxidesWater vaporCarboxylic acid
An apparatus for producing metal oxide comprising (a) a water vapor generator for generating a water-vapor containing gas with a desired partial pressure of water vapor; and (b) heating equipment for heating a metal salt of a carboxylic acid, the metal salt of the carboxylic acid being disposed in a sample vessel, to a predetermined temperature in a water-vapor-containing gas which is introduced from said water vapor generator.
Owner:RIGAKU CORP
Bell column downtube, reactors utilizing same and related methods
InactiveUS20130020236A1Laboratory glasswaresLiquid hydrocarbon mixture productionChemical reactionEngineering
Reactors for carrying out a chemical reaction, as well as related components, systems and methods are provided. In accordance with one embodiment, a reactor is provided that includes a furnace and a crucible positioned for heating by the furnace. A downtube is disposed at least partially within the interior crucible along an axis. At least one structure is coupled with the downtube and extends substantially across the cross-sectional area of the interior volume taken in a direction substantially perpendicular to the axis. A plurality of holes is formed in the structure enabling fluid flow therethrough. The structure coupled with the downtube may include a lower body portion and an upper body portion coupled with the lower body portion, wherein the plurality of holes is formed in the lower body portion adjacent to, and radially outward from, a periphery of the upper body portion.
Owner:BATTELLE ENERGY ALLIANCE LLC
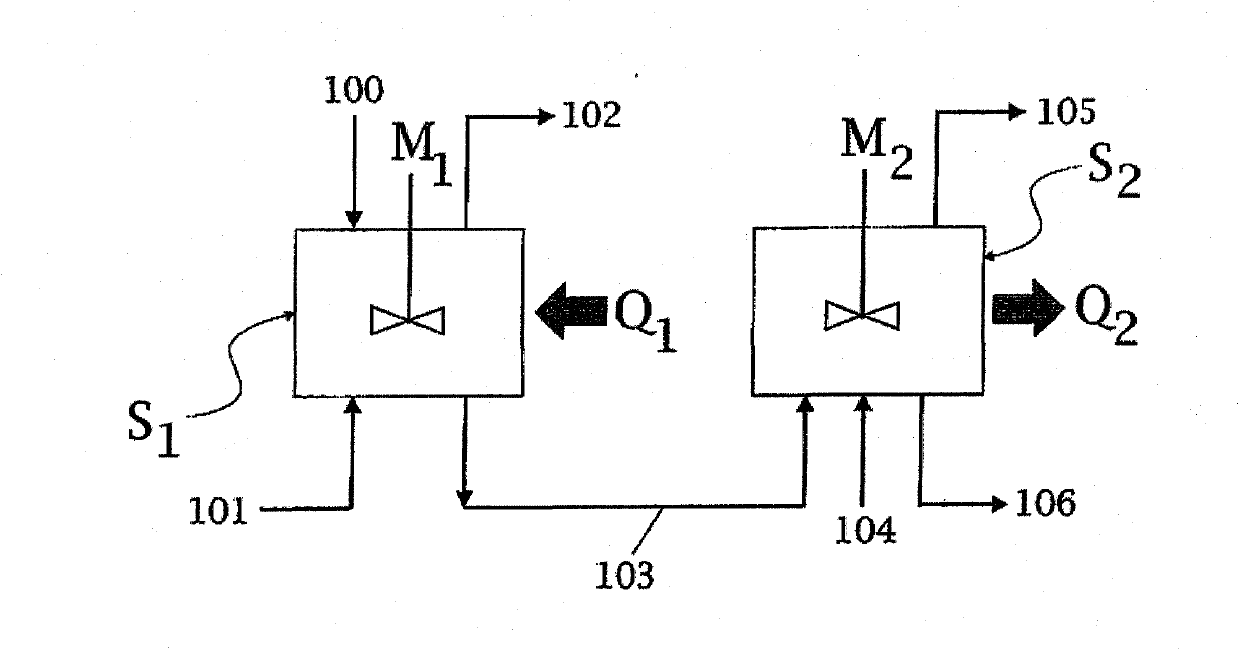
Process for Producing High-Quality Melamine from Urea
InactiveUS20110054169A1Good effectIncrease heat transfer rateProcess control/regulationOrganic chemistryReaction zoneHigh pressure
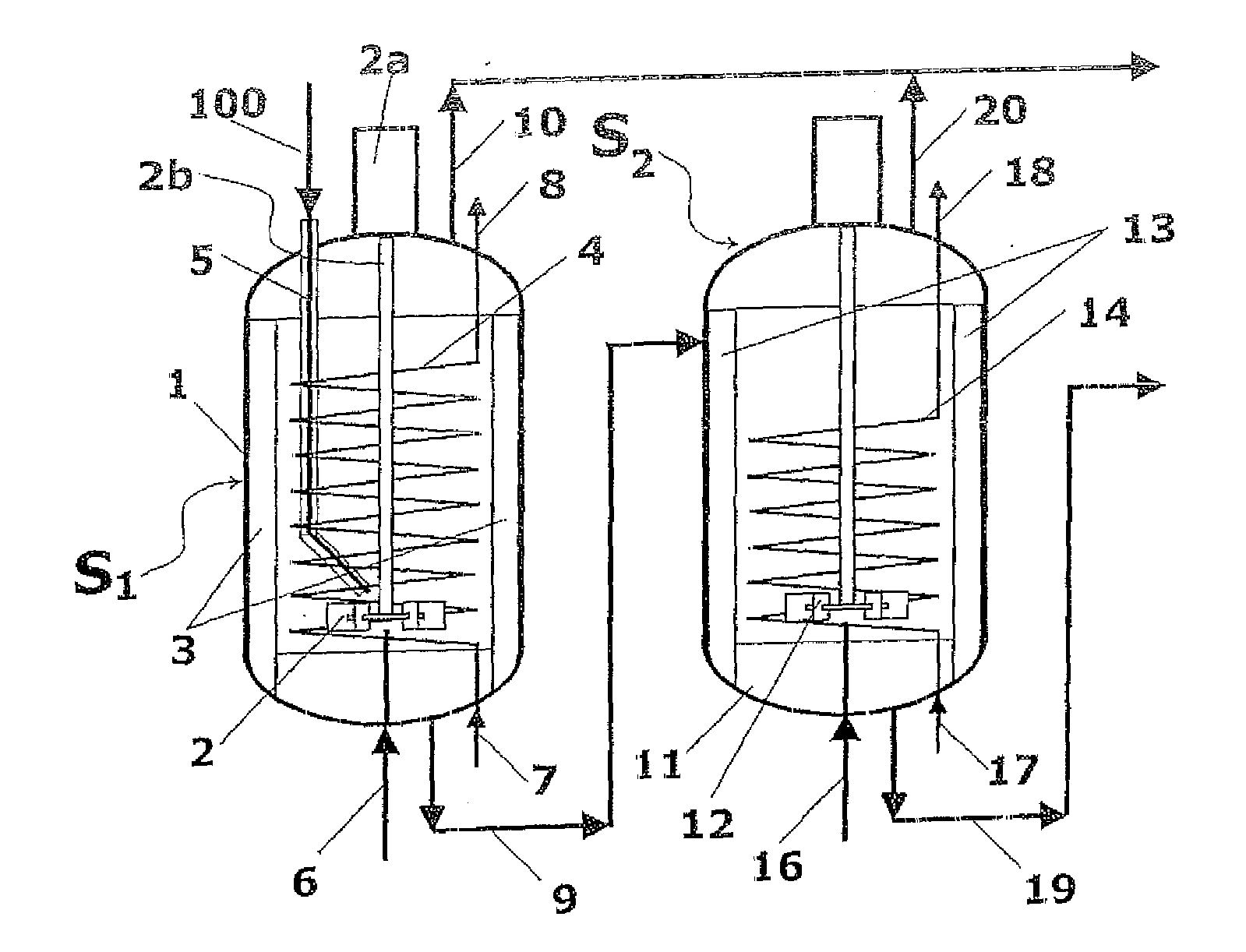
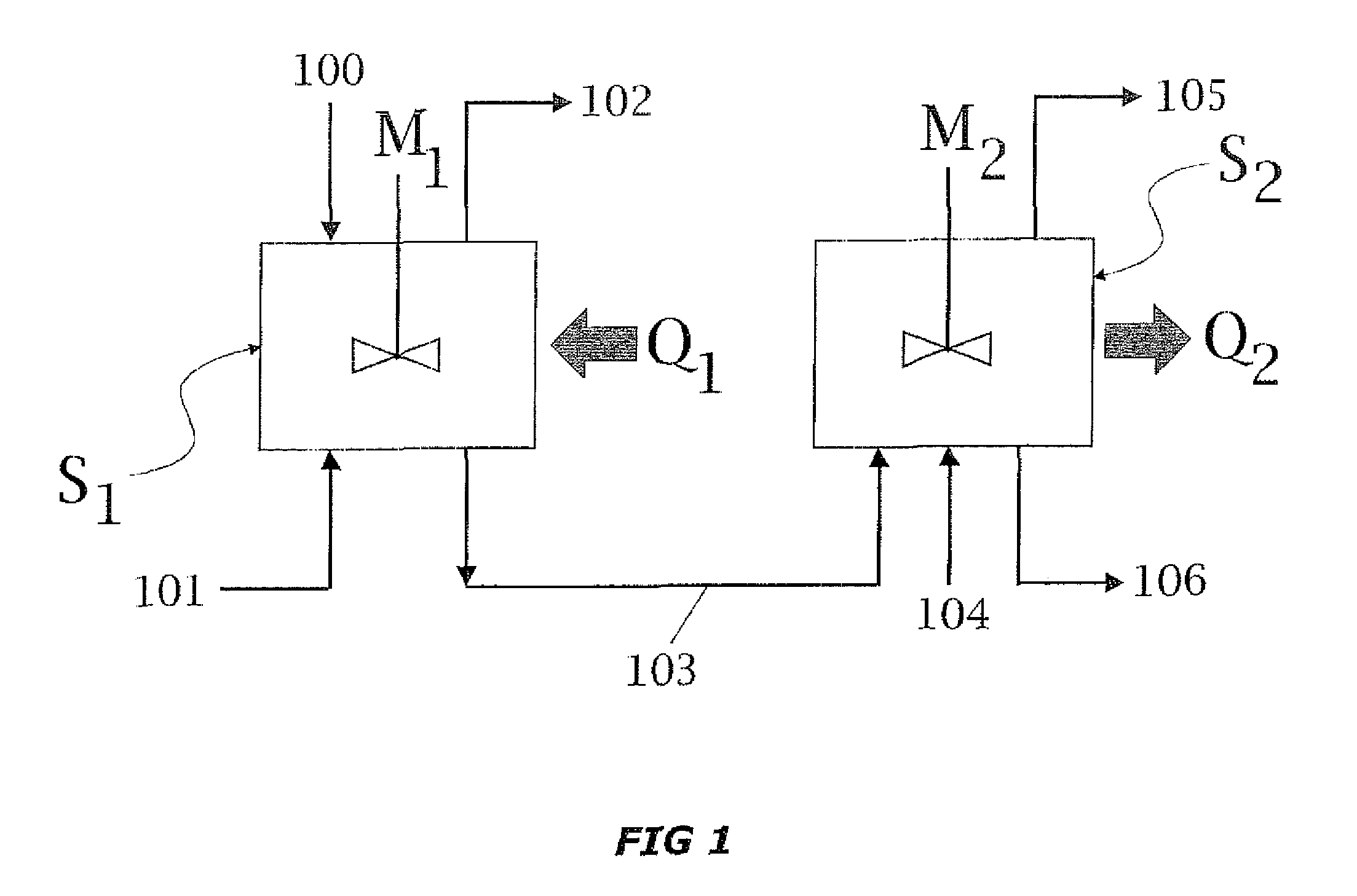
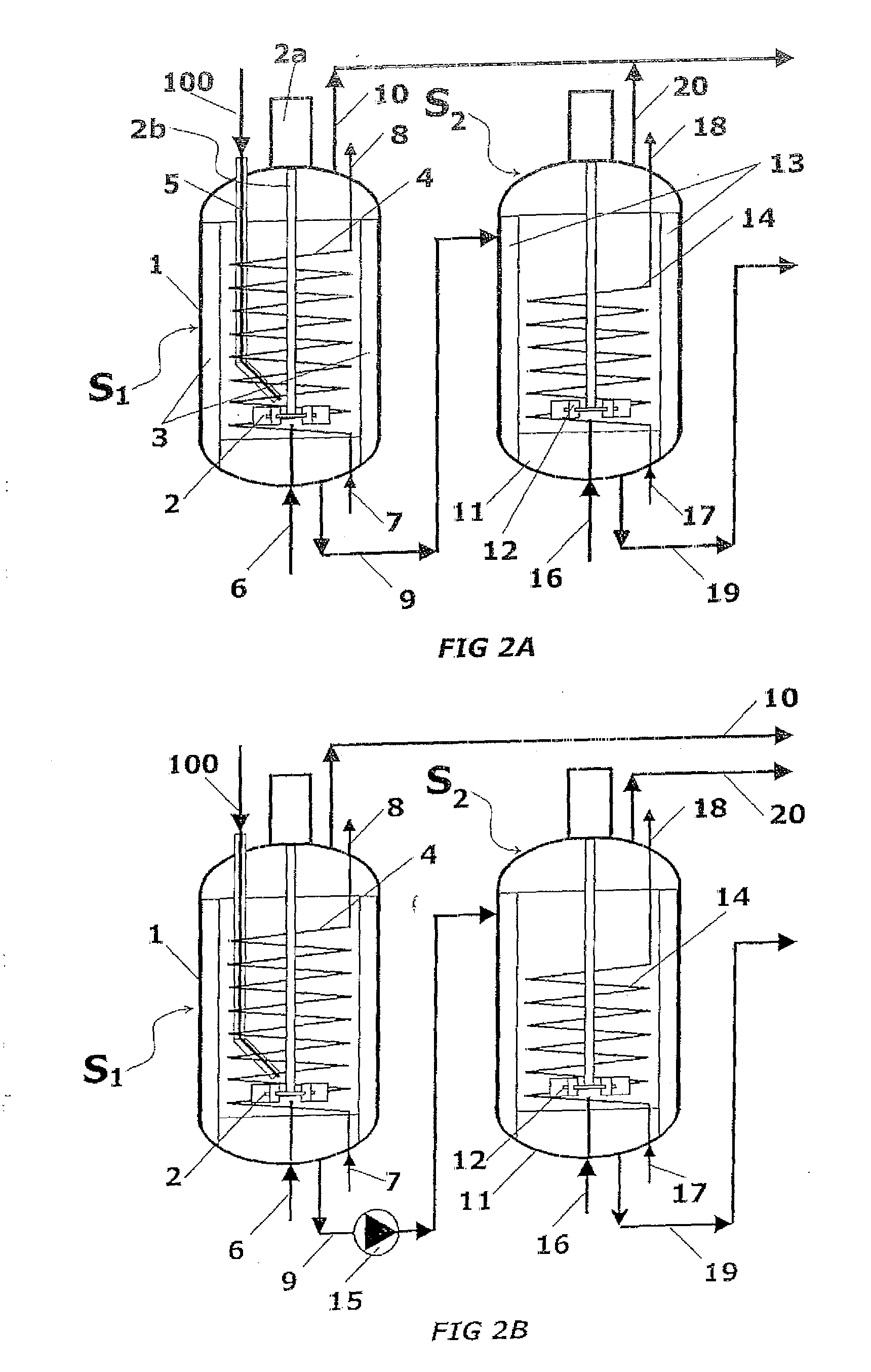
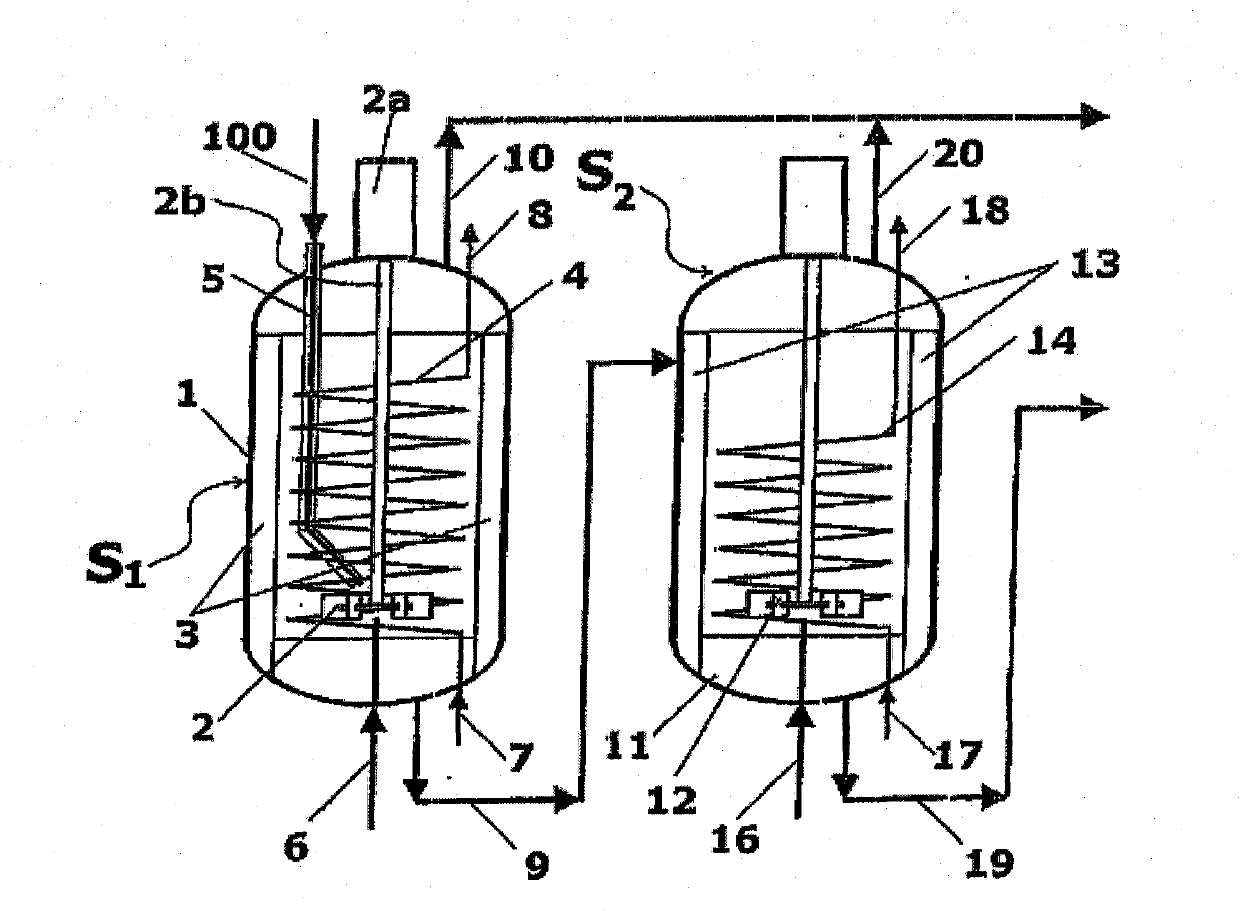
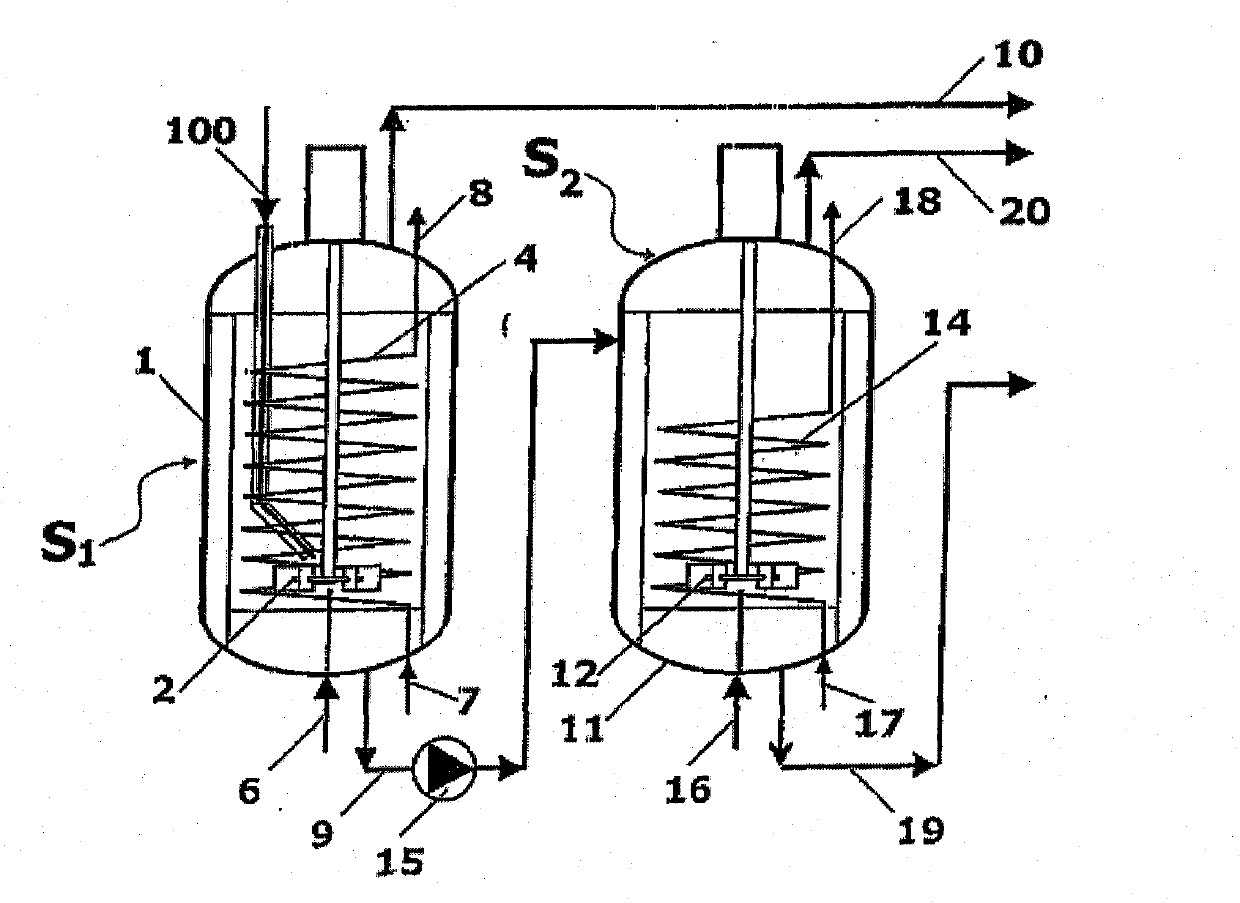
A process for high-pressure, liquid phase conversion of urea into melamine is disclosed, where molten urea is fed to a first reaction zone (S1) where the melamine melt is under mechanical agitation, and a heat input (Q1) is provided to maintain the endothermic reaction, and the liquid is then passed to a second reaction zone (S2) kept at a lower temperature and where further agitation is provided. Embodiments of plants adapted to carry out the process are also disclosed, including multiple stirred reactors in cascade and a single reactor with multiple internal compartments defining said first and second reaction zones.
Owner:CASALE SA
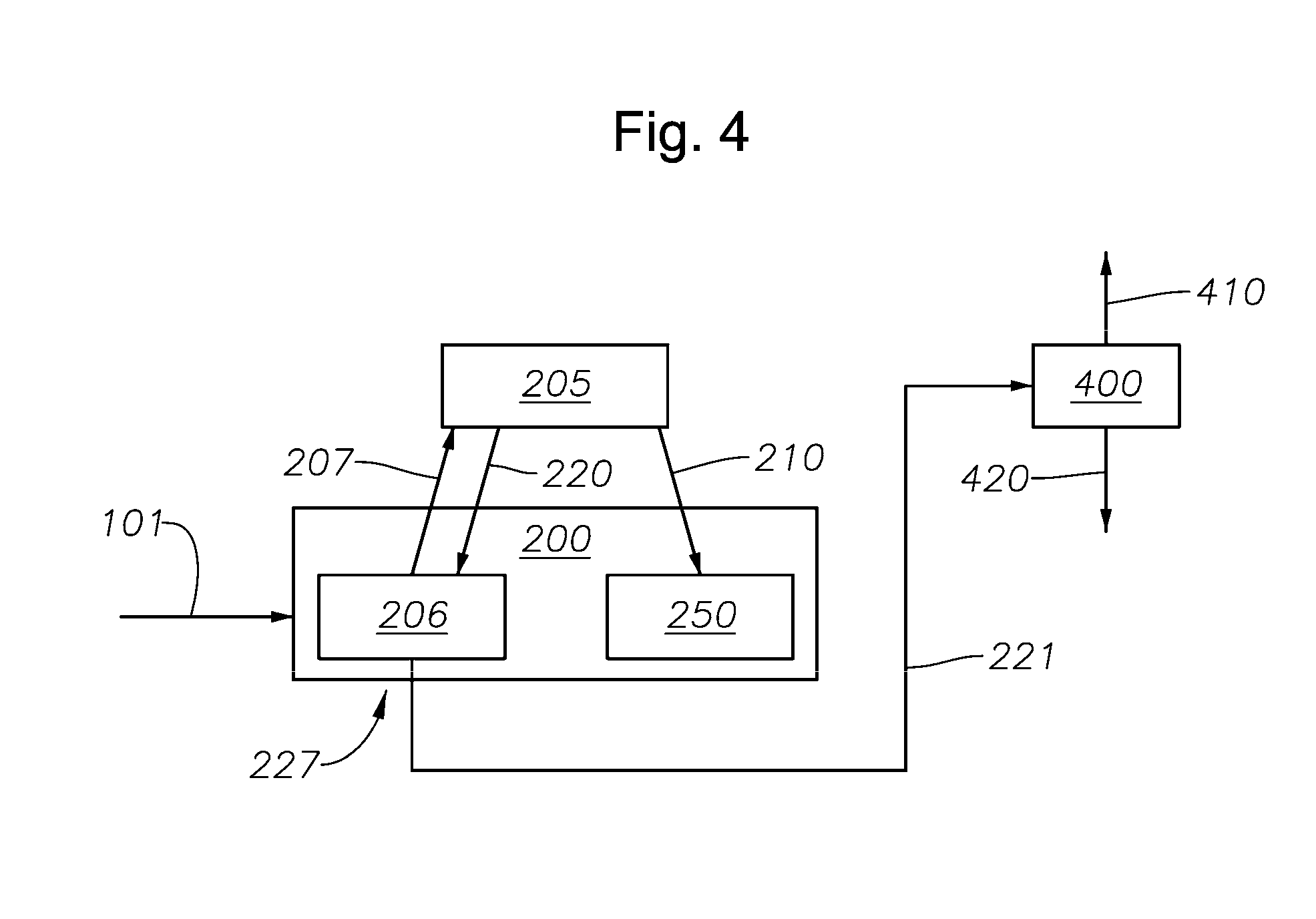
Hydrogen Sulfide Production Process and Related Reactor Vessels
The present invention discloses a hydrogen sulfide reactor vessel with an external heating system that is conductively and removably attached to an exterior portion of the reactor vessel. Also disclosed are processes for producing hydrogen sulfide utilizing the reactor vessel.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Process for producing high-quality melamine from urea
InactiveCN102015663ADelayed expansionSpeed up heat exchangeProcess control/regulationOrganic chemistryFluid phasePhysical chemistry
A process for high-pressure, liquid phase conversion of urea into melamine is disclosed, where molten urea is fed to a first reaction zone (S1 ) where the melamine melt is under mechanical agitation, and a heat input (Q1 ) is provided to maintain the endothermic reaction, and the liquid is then passed to a second reaction zone (S2) kept at a lower temperature and where further agitation is provided. Embodiments of plants adapted to carry out the process are also disclosed, including multiple stirred reactors in cascade and a single reactor with multiple internal compartments defining said first and second reaction zones.
Owner:UREA CASALE SA
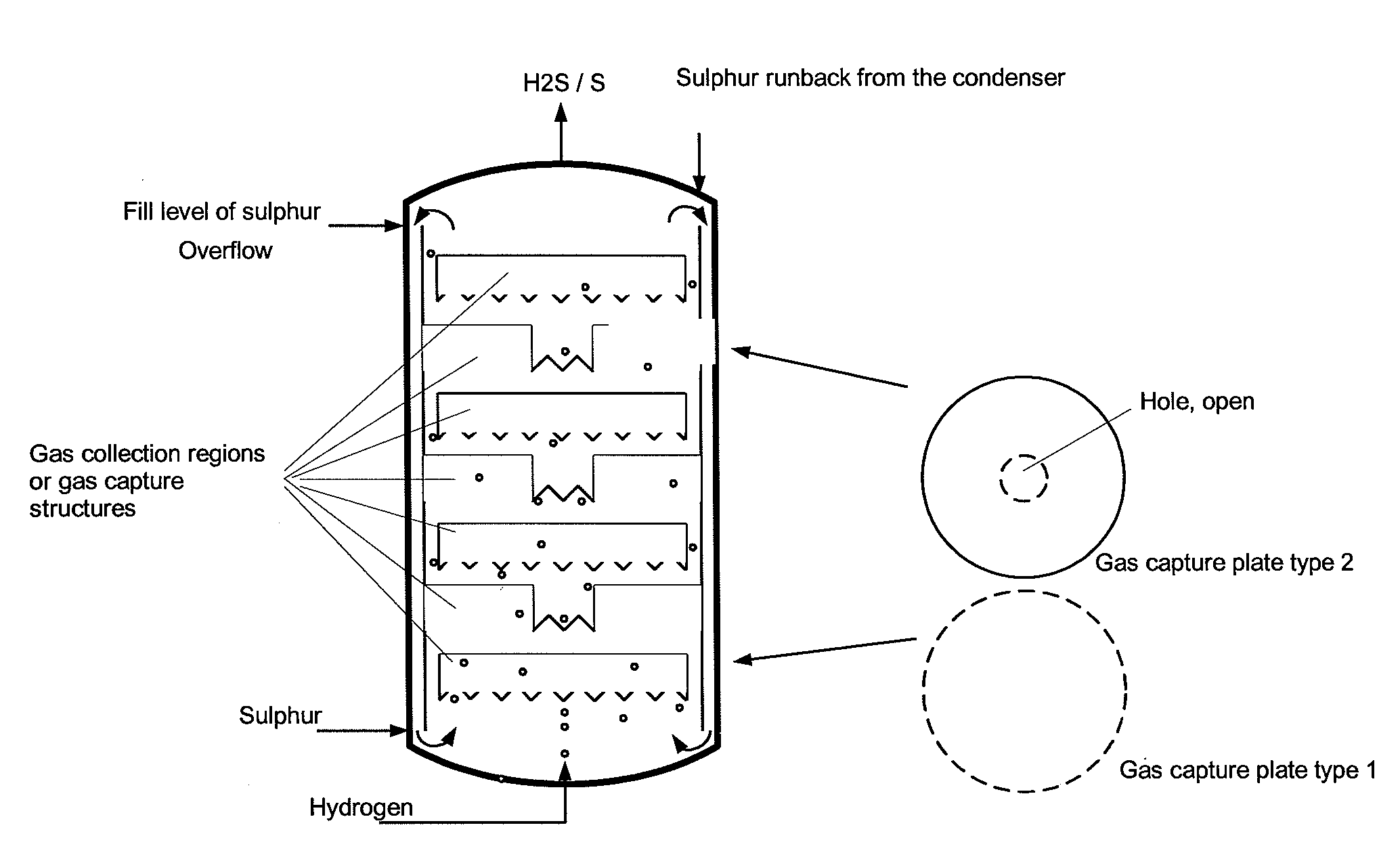
Reactor and process for preparing hydrogen sulphide
InactiveCN104603048AHigh purityImprove hydrogen conversionHydrogen sulfidesHigh temperature liquid-gas reactionRefluxProduct gas
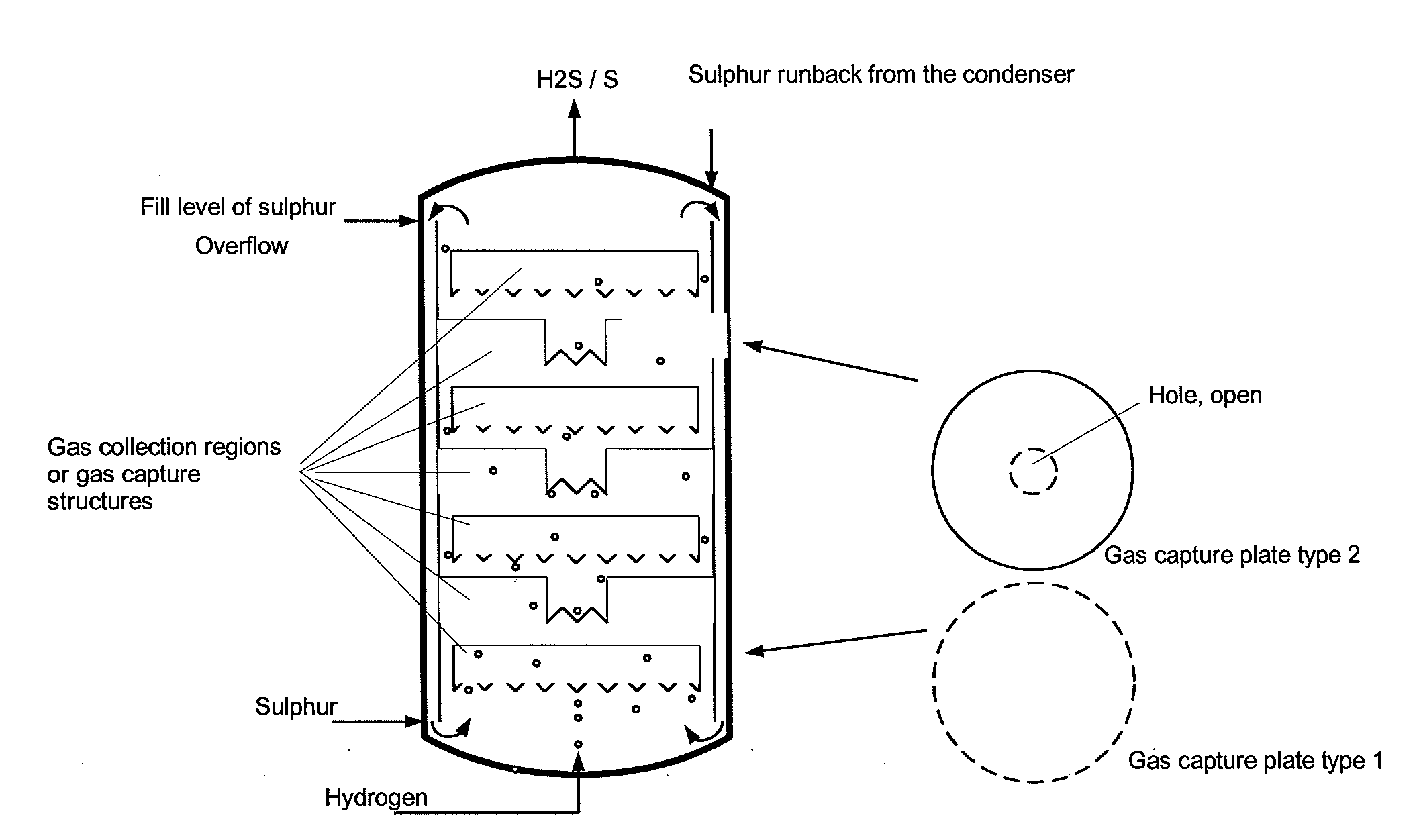
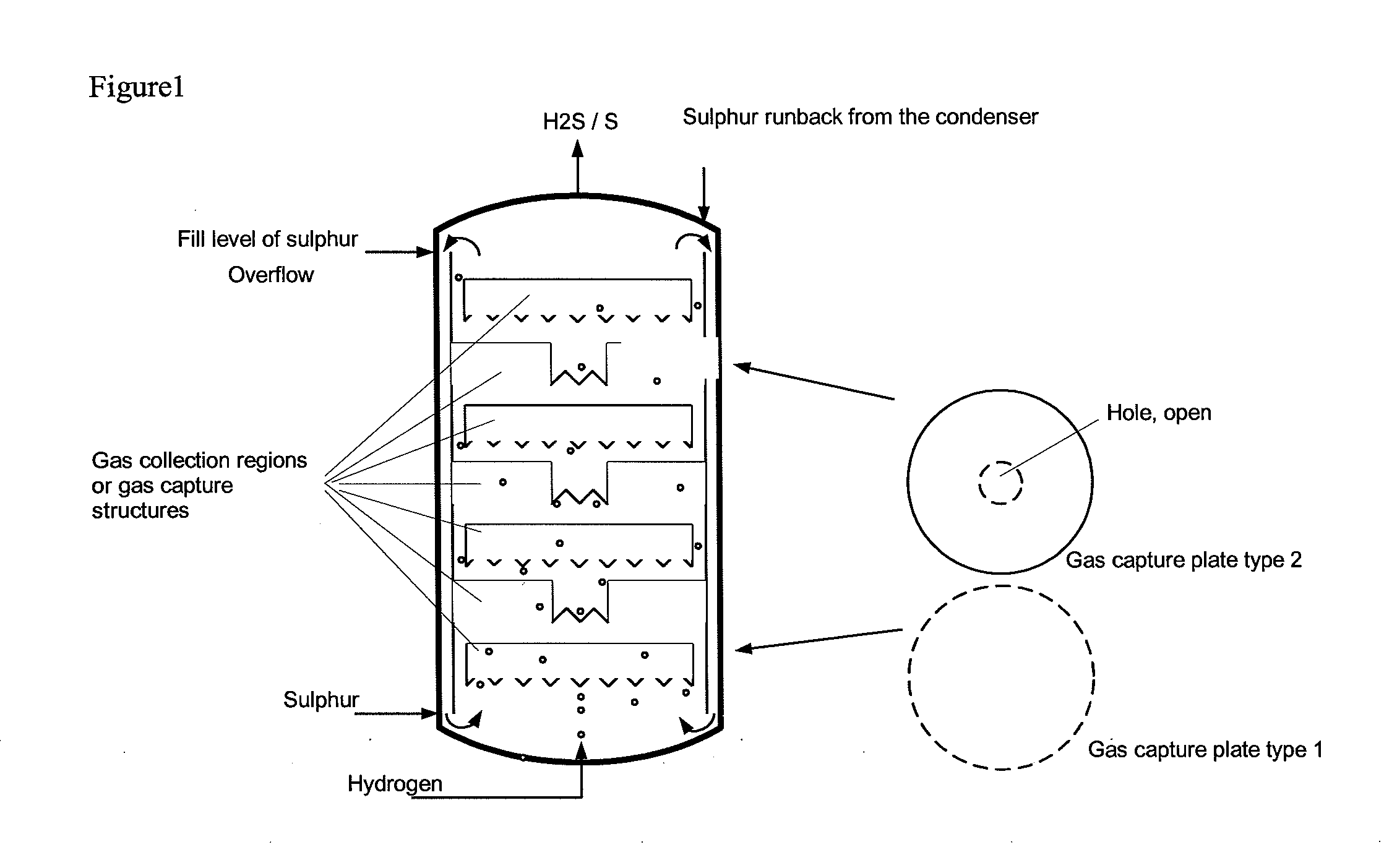
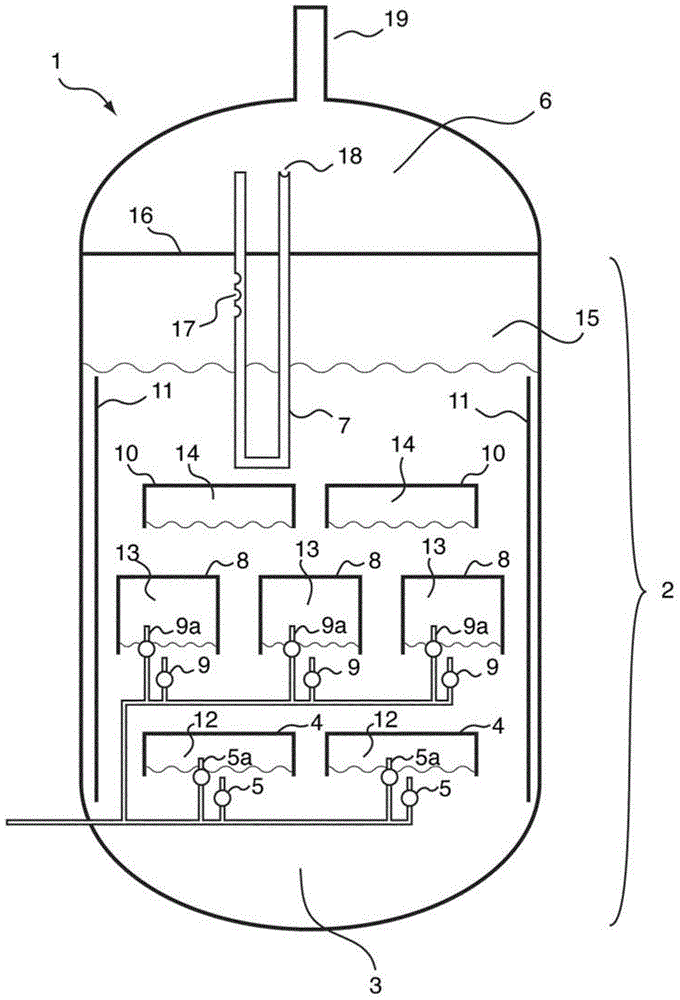
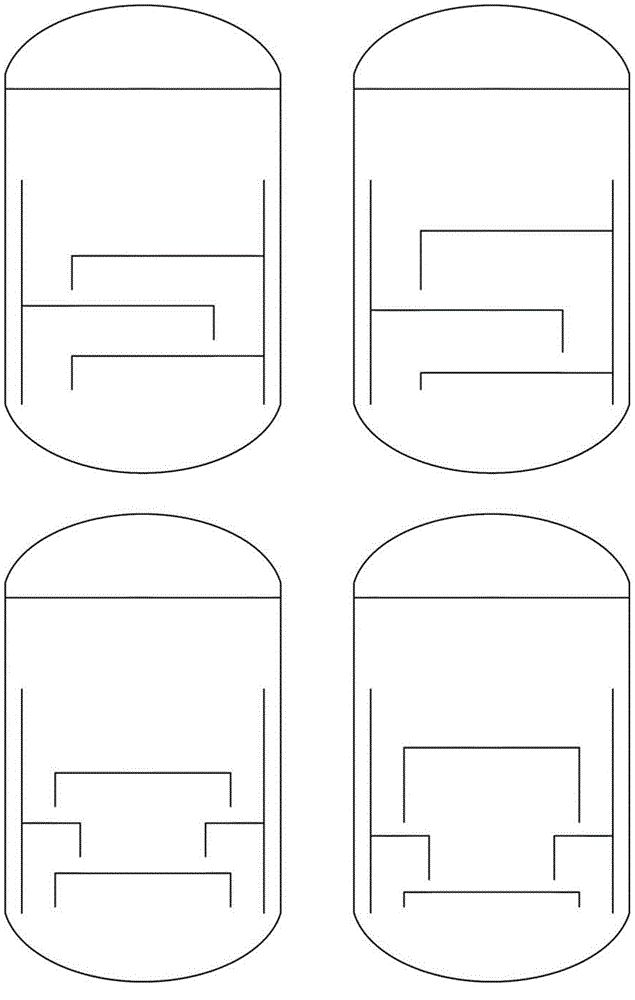
The reactor comprises: a lower reactor region (2) for accommodating a sulfur melt (3); non-pressure-bearing first caverns (4) for temporary accommodation of a product gas mixture that is formed by exothermic reaction and comprises hydrogen sulfide, sulfur and hydrogen; non-pressure bearing second caverns (8) arranged above the first caverns and for temporary accommodation of the product gas mixture formed in the first caverns and for formation of further hydrogen sulfide by exothermic reaction of sulfur and hydrogen to form a product gas mixture; and a gas collecting region (6). The reactor comprises: a lower reactor region (2) for accommodating a sulfur melt (3); non-pressure-bearing first caverns (4) for temporary accommodation of a product gas mixture that is formed by exothermic reaction and comprises hydrogen sulfide, sulfur and hydrogen, and a supply device for controlled supply of pressurized gaseous hydrogen per first cavern; non-pressure bearing second caverns (8) arranged above the first caverns and suitable for temporary accommodation of the product gas mixture formed in the first caverns and for formation of further hydrogen sulfide by exothermic reaction of sulfur and hydrogen to form a product gas mixture; and a gas collecting region (6) for accommodating the product gas mixture at elevated temperature and elevated pressure relative to standard conditions. One of the second caverns comprises a supply device suitable for controlled supply of pressurized gaseous hydrogen. The reactor further comprises: non-pressure-bearing third caverns arranged above the second caverns; non-pressure-bearing installed devices suitable for continuous transfer of a total amount of product gas mixture formed in the lower reactor region to the gas collecting region, where a catalyst is present in the installed devices suitable for reacting sulfur and hydrogen still present in the product gas mixture to form hydrogen sulfide; an inner wall that allows continuous circulation of the sulfur melt according to an airlift pump principle in the course of operation of the reactor with involvement of a space between an outer reactor wall and the inner wall; a reflux condenser suitable for condensation of the sulfur present in the product gas mixture; and an input line suitable for transport of the product gas mixture from the gas collecting region to the reflux condenser and a return line suitable for return of the condensed sulfur to the reactor. One of the second or third caverns has a greater volume than each of the first caverns, and / or one of the second or third caverns has lower heat removal for construction reasons than each of the first caverns. The installed devices are arranged such that, the installed devices are present in thermal contact with the sulfur melt after sufficient filling of the lower reactor region with the sulfur melt. The catalyst is cooled by transfer of heat to the sulfur melt if the installed devices contain the catalyst. An independent claim is included for a process for preparing hydrogen sulfide by exothermic reaction of sulfur with hydrogen at elevated temperature and elevated pressure relative to standard conditions to form a product gas mixture comprising hydrogen sulfide and sulfur.
Owner:EVONIK OPERATIONS GMBH
Devices and systems for producing silicon
ActiveCN108367928AReduce energy costsReduce economic costsCatalytic crackingHigh temperature liquid-gas reactionSiliconChemistry
The present disclosure provides devices and systems that utilize concurrent and countercurrent exchange platforms to produce purified silicon.
Owner:MILWAUKEE SILICON LLC
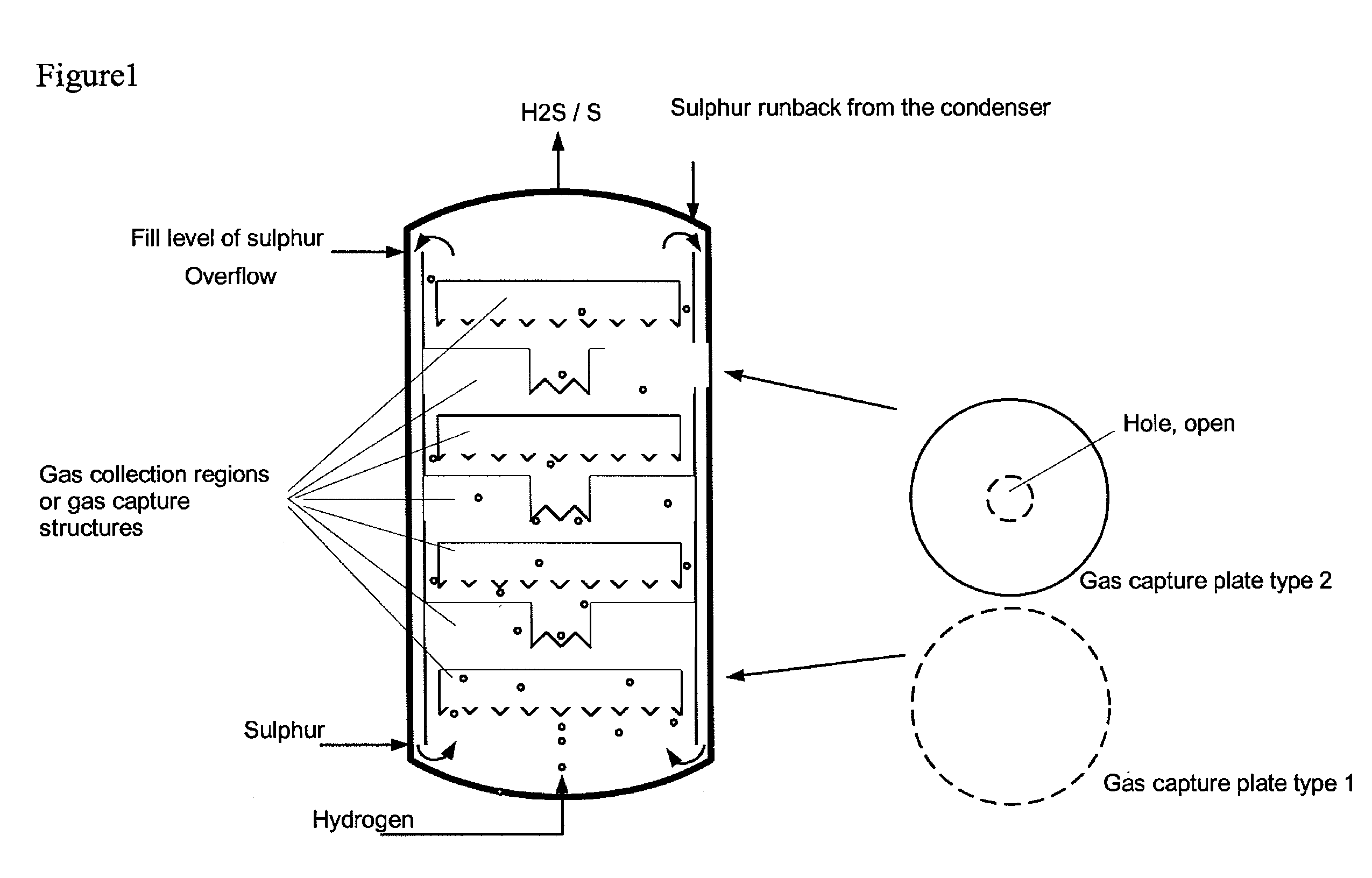
Reactor and process for preparing hydrogen sulphide
InactiveCN104583116AHigh purityCompact designHydrogen sulfidesHigh temperature liquid-gas reactionProduct gasGaseous hydrogen
A reactor (1) has lower reactor region (2) for accommodating sulfur melt (3), non-pressure-bearing caverns (4) for accommodation of product gas mixture which forms in exothermic reaction and contains sulfur, supply device (5,5a) for controlled supply of pressurized gaseous hydrogen per cavern, gas-collecting region (6) for accommodating the product gas mixture, non-pressure-bearing installed device(s) (7) for transfer of the total amount of product gas mixture formed in the lower reactor region to the gas-collecting region and catalyst in the non-pressure-bearing installed device(s). A reactor has lower reactor region for accommodating sulfur melt, non-pressure-bearing caverns for temporary accommodation of product gas mixture which forms in exothermic reaction and comprises hydrogen sulfide, sulfur and hydrogen, at least one supply device for controlled supply of pressurized gaseous hydrogen per cavern, gas-collecting region for accommodating the product gas mixture at elevated temperature and elevated pressure with respect to standard conditions, non-pressure-bearing installed device(s) for continuous transfer of the total amount of product gas mixture formed in the lower reactor region to the gas-collecting region and catalyst in the non-pressure-bearing installed device(s), for reaction of sulfur and hydrogen maintained in the product gas mixture to hydrogen sulfide. An independent claim is included for preparation of hydrogen sulfide.
Owner:EVONIK OPERATIONS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com