Nucleotides, viral vectors and their applications and RNAi pharmaceutical preparations
A technology of viral vectors and pharmaceutical preparations, applied in the field of biomedicine, can solve problems such as affecting the efficiency of RNAi and the amount of shRNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of shRNA vectors expressed by different promoters
[0045] 1. Promoter fragment digestion
[0046] After the gene sequences of SEQ ID NOs: 1-6 were synthesized by the gene, they were digested with endonuclease respectively, and the enzyme digestion system was as follows:
[0047]
[0048] The pAAV2 plasmid was digested with the same method, and the target band was recovered after 1 h of reaction at 37°C.
[0049] 2. Construction of recombinant vector
[0050] Take the fragment and the vector cut by the above-mentioned enzyme, respectively, and connect them. The connection system is as follows:
[0051]
[0052] After the ligation system was reacted at 25°C for 10 min, the ligation product was taken to transform stbl3 competent cells. Mix well according to the following reaction system, place on ice for 20 min, place at room temperature for 10 min, add 500 μL of anti-anti-LB at 37°C, incubate at 200 rpm for 40 min, then centrifuge at 5000 ...
Embodiment 2
[0079] Example 2 Screening of efficient RNAi drugs by luciferase reporter system
[0080] 1. Culture of mammalian cells (adherent)
[0082] 1) Prepare warm water at 37°C-38°C in advance, take out the cells to be resuscitated from the liquid nitrogen tank, fix them with ophthalmic surgical forceps, and quickly place them in water to ensure that the cryovials are completely immersed in water, so that they are evenly heated until frozen. The cells in the deposit tube are completely thawed;
[0083] 2) Disinfect the cryopreservation tube with alcohol;
[0084] 3) Pipette 5mL of cell culture-based T25 cell culture flask in advance with a pipette, and then use a new pipette to transfer the thawed cells into the cell flask and gently pipette again;
[0085] 4) Cover the cell flask and place the cell flask in a cell incubator at 37°C, 5% CO 2 static culture;
[0086] 5) After about 6-8 hours (depending on different cells), replace with fresh medium to el...
Embodiment 3
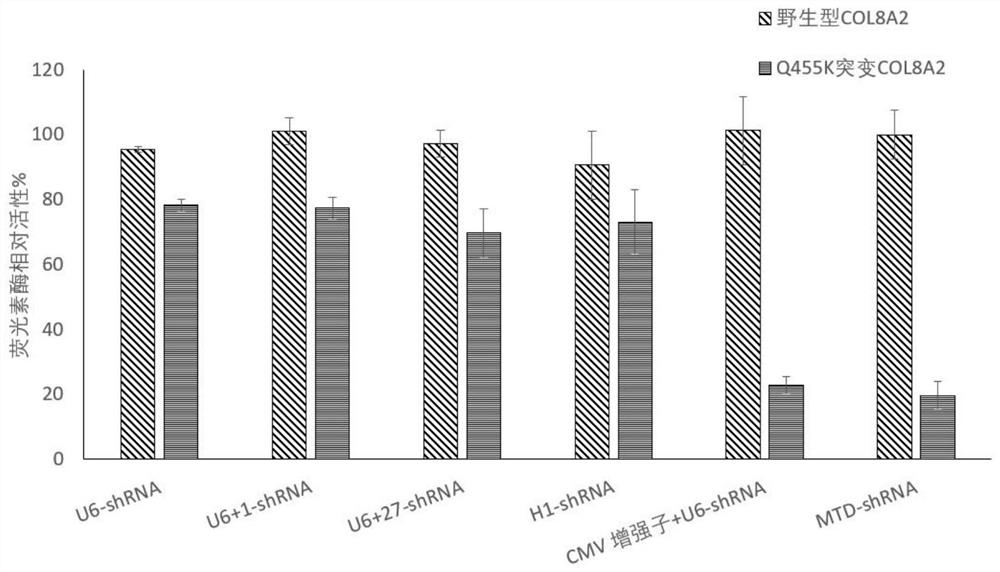
[0109] Example 3 RNAi drug treatment inhibits mutation-specific COL8A2 gene expression
[0110] 1. 293 cell transfection:
[0111] The method is the same as before.
[0112] 2. Detection of COL8A2 RNA level by reverse transcription fluorescent quantitative PCR
[0113] 1. The reverse transcription reaction system is as follows:
[0114]
[0115] Reverse transcription reaction conditions: 37°C for 1 h, 75°C for 10 min.
[0116] 2. Real-time reaction system
[0117] 1) Detection primers and internal reference primers for the target gene
[0118] COL8A2: 5'-GGCGGGCTATGCCCCAGTGAAGTAC-3'(sense)
[0119] 5’-CTGGCTTTCCCATGCCTGGTTTTCC-3’(antisense)
[0120] GAPDH: 5'-GGAAGGTGAAGGTCGGAGTCAACGG-3'(sense)
[0121] 5’-CTCGCTCCTGGAAGATGGTGATGGG-3’(antisense)
[0122] 2) Reaction system
[0123]
[0124] 3) Reaction procedure:
[0125]
[0126] 3. AAV infected 293 cells
[0127] Recombinant virus AAV-shRNA control, AAV-shRNA drug with MOI=1×10 4 The multiplicity of infect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com