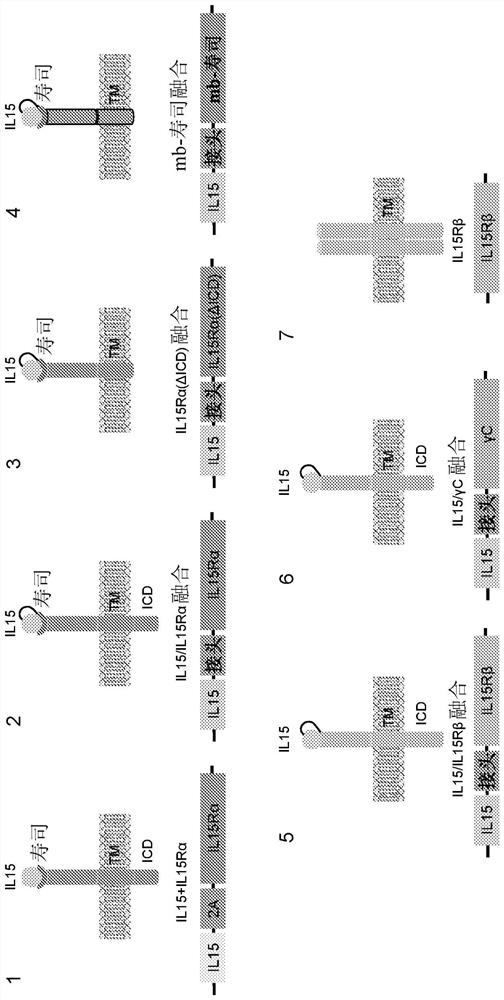
Engineered immune effector cells and use thereof
A technology of hematopoietic cells and cells, applied in the field of engineered immune effector cells and their uses, can solve problems such as unmet improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0346] The following examples are provided for illustration, not limitation.
[0347] Example 1 - Materials and methods
[0348] In order to efficiently select and test suicide systems under the control of different promoters combined with different safe harbor locus integration strategies, the applicant's proprietary hiPSC platform, which is capable of single cell passage and high-throughput 96-based Flow cytometric sorting of well plates to derive clonal hiPSCs with single or multiple gene regulation.
[0349] Maintenance of hiPSCs in small molecule culture: hiPSCs are conventionally passaged as single cells after the culture confluency reaches 75%-90%. For single cell dissociation, hiPSCs were washed once with PBS (Mediatech) and treated with Accutase (Millipore) for 3-5 min at 37°C, followed by pipetting to ensure single cell dissociation. The single cell suspension was then mixed with an equal volume of conventional medium, centrifuged at 225 xg for 4 minutes, resuspend...
example 2
[0352] Example 2 - CD38 knockout in iPSCs using CRISPR / Cas9-mediated genome editing
[0353] Alt- S.p.Cas9 D10A Nickase 3NLS (100μg) and Alt- CRISPR-Cas9 tracrRNA was purchased from IDT (Coralville, Iowa) and used for iPSC targeted editing. For biallelic knockout of CD38 in iPSCs using Cas9 nickase, the screened and identified targeting sequence pairs designed for gNA (i.e., gD / RNA or guide polynucleotide) are listed in Table 3 (1A and 1B, 2A and 2B, 3A and 3B):
[0354] Table 3: Targeting sequences specific to the CD38 locus for CRISPR / Cas9 genome editing:
[0355] Exon / Chr# targeting sequence PAM cleavage site SEQ ID NO: CD38-gNA-1A 1 / 4 TTGACGCATCGCGCCAGGA CGG 15,778,604 1 CD38-gNA-1B 1 / 4 ATTCATCCTGAGATGAGGT GGG 15,778,646 2 CD38-gNA-2A 1 / 4 ACTGACGCCAAGACAGAGT TGG 15,778,485 3 CD38-gNA-2B 1 / 4 CTGGTCCTGATCCTCGTCG TGG 15,778,520 4 CD38-gNA-3A 1 / 4 TCCTAGAGAGCCGGCAGCA GGG 15,778,459 5 CD38-...
example 3-CD3
[0357] Example 3-CD38 - / - Validation of iPSCs and derived cells
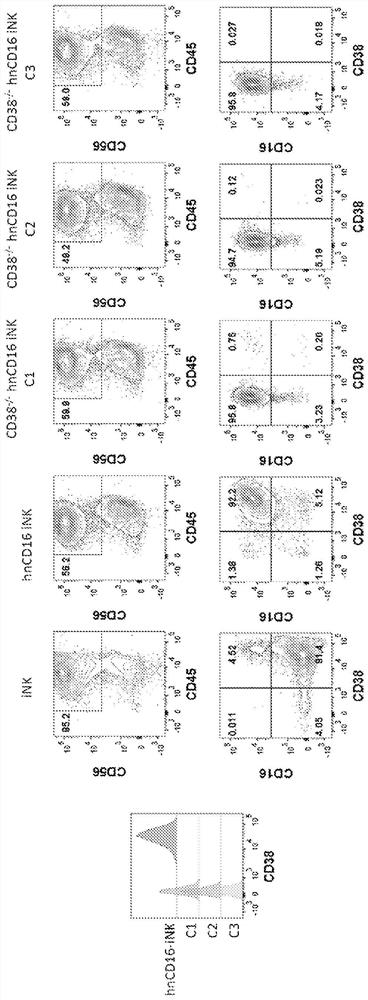
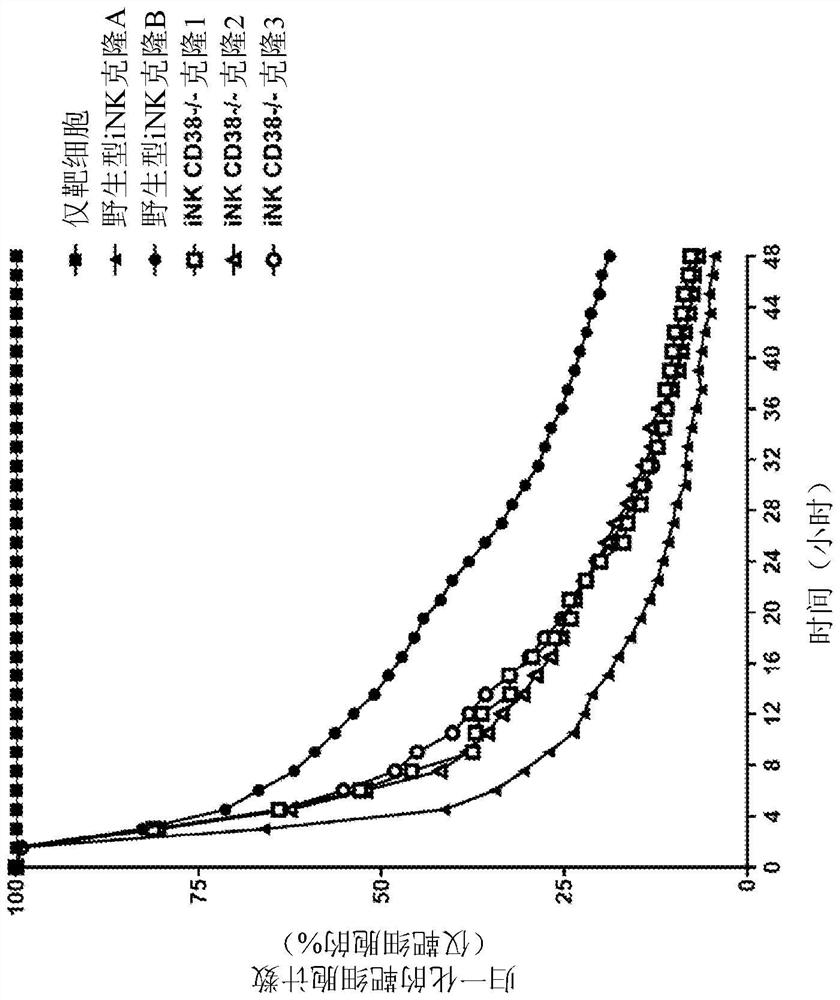
[0358] CD38 is known to be expressed at specific cell stages and plays a key role in effector cells. During hematopoiesis, CD38 in CD34 + Expressed on stem cells and lineage specialized progenitors of lymphoid, erythroid and myeloid, and also during the final stages of maturation of effector cells such as T cells and NK cells. Thus, prior to this application, it was unknown and worrisome whether iPSCs comprising a CD38 knockout would develop normally when subjected to directed differentiation conditions, and whether the resulting effector cells would function as CD38 expression profiles and functions. effect. CD38-knockout iPSCs containing biallelic knockout of CD38 surprisingly maintained their ability to differentiate into derivative cells. In one of the illustrations, three engineered iPSC clones gene-edited to have a biallelic disruption of the CD38 gene and hnCD16 were differentiated into derived NK cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com