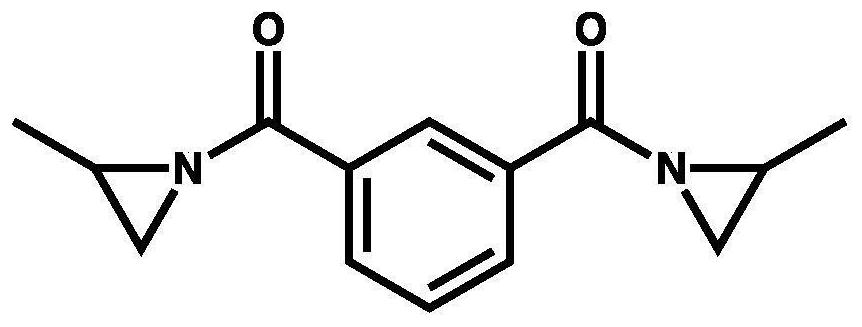
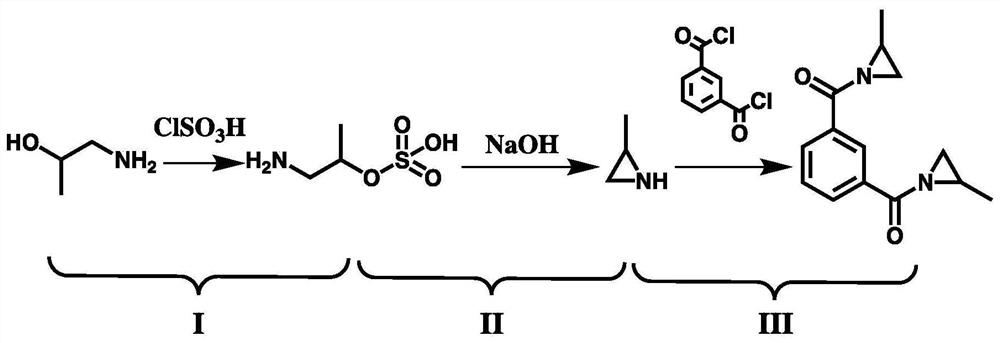
Synthetic method of aromatic aziridine cross-linking agent
A technology of aziridine crosslinking agent and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of increased waste discharge, cumbersome sulfate ester synthesis process, and increased risk, so as to save energy and cost and have a good industrialization prospect , the effect of reducing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
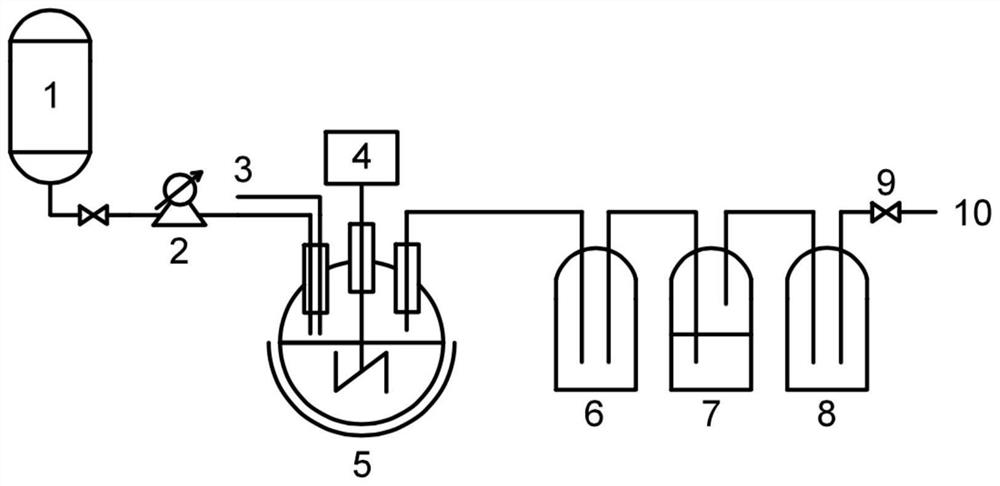
[0030] [Example 1] The structural design of the synthetic reactor of aminoisopropylsulfate
[0031] The structure of the reactor for the synthesis of aminoisopropyl sulfate refers to the attached image 3 . Among them, 1-diluted chlorosulfonic acid storage tank, 2-feed pump, 3-nitrogen inlet, 4-stirring motor, 5-jacketed reactor, 6-front protection tank, 7-acid mist absorption tank , 8-rear protection tank, 9-vacuum regulating valve, 10-vacuum inlet.
Embodiment 2
[0032] [embodiment 2] the synthesis of amino isopropyl sulfate
[0033] Transfer 150.0g of 1-amino-2-propanol and 1000mL of diethyl ether into the reactor, start stirring to make them fully mixed. Put in a circulating water bath to cool the system temperature to about 8°C, and at the same time, mix 233.0g of chlorosulfonic acid and 500mL of diethyl ether evenly and transfer it to a liquid storage tank for use; blow nitrogen into the reactor and adjust the vacuum to -0.008MPa; Slowly pump the diluted chlorosulfonic acid solution into the reactor with vigorous stirring, and maintain the stability of the system temperature by adjusting the feeding speed; after the feeding is completed, maintain the temperature and continue to stir for 0.5h; after the reaction, filter the reaction solution The insoluble matter was collected and fully rinsed with ether; after vacuum drying, 283.2 g of white powder product aminoisopropyl sulfate was obtained, with a yield of 85.4%.
Embodiment 3
[0034] [embodiment 3] the synthesis of amino isopropyl sulfate
[0035] Transfer 132.0g of 1-amino-2-propanol and 1000mL of cyclohexane into the reactor, start stirring to make them fully mixed. Pass through a circulating water bath to cool the system temperature to about 4°C. At the same time, mix 209.0g of chlorosulfonic acid and 500mL of cyclohexane evenly and transfer it to a liquid storage tank for use; pass nitrogen into the reactor and adjust the vacuum to -0.014 MPa; slowly pump the diluted chlorosulfonic acid solution into the reactor with vigorous stirring, and maintain the stability of the system temperature by adjusting the feeding speed; after the feeding is completed, keep the temperature and continue to stir for 1h; The insoluble matter was collected by filtration and fully rinsed with cyclohexane; after vacuum drying, 285.4 g of white powder product aminoisopropyl sulfate was obtained, with a yield of 92.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com