Method for preparing dithiocoumarin benzothiophene compound through platinum catalysis
A technology of dithiocoumarin and benzothiophene, applied in the direction of organic chemistry, etc., can solve the problems of low reaction efficiency and many reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The product of Preparation 4a: Example 1
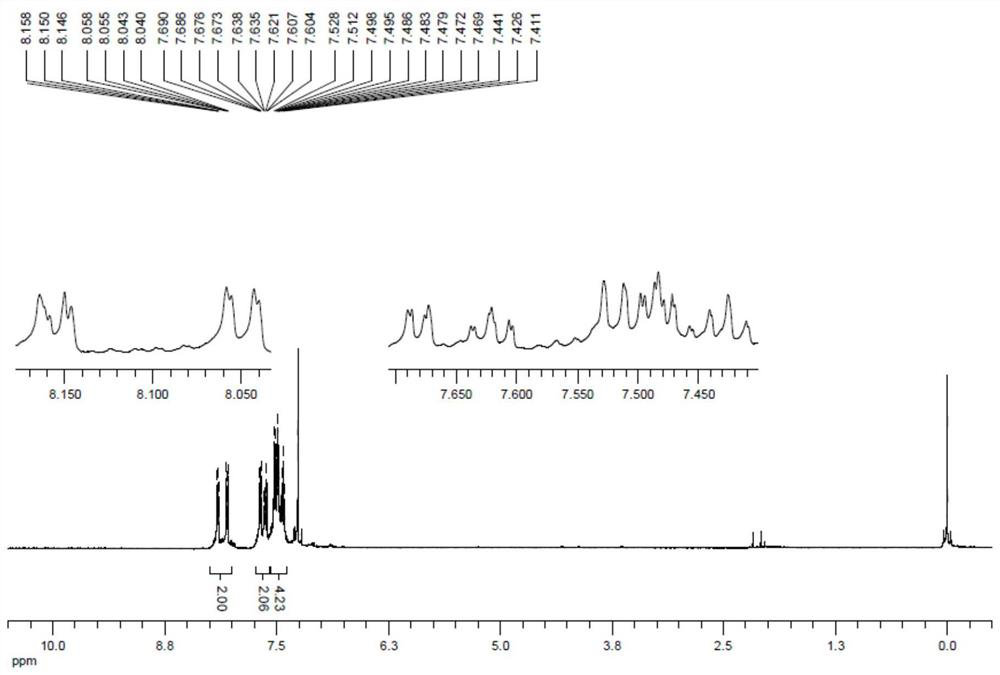
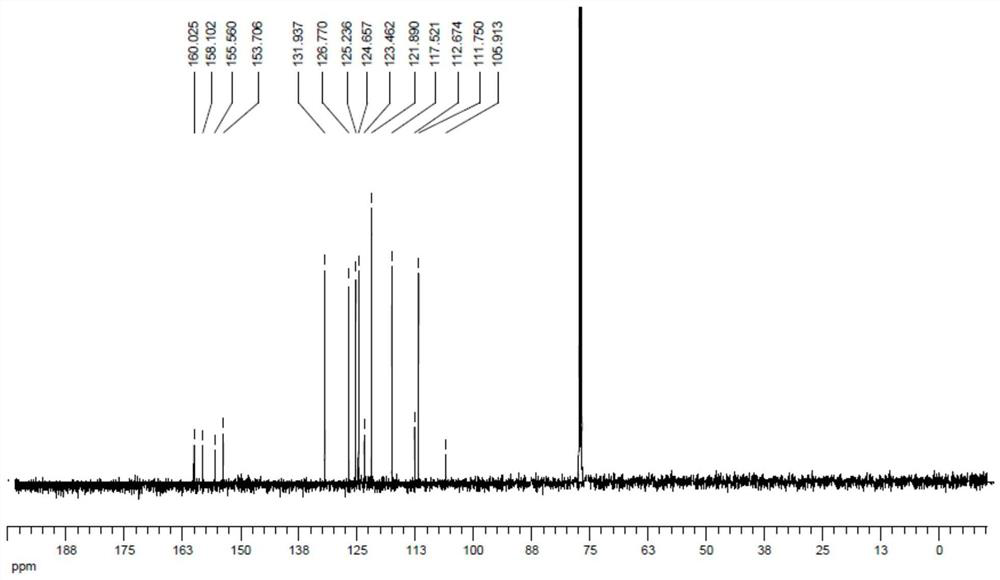
[0041] At room temperature in a round bottom flask was added 25mL of so 5mmol2- mercapto cinnamic acid, 6mmol carbon disulfide, and 2-bromothiophenol 5mmol then successively added 15mL acetonitrile, 0.5mmol platinum chloride and 10mmol sodium in ethanol, the reaction was stirred at 80 ℃ 8 hours. After cooling, to the system was added 20mL saturated sodium chloride aqueous solution, and extracted 3 times with ethyl acetate, 20mL each, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was evaporated, 200-300 mesh silica gel column chromatography to give coumarin and the dithiophosphoric benzothiophene compound 4a (1278mg, 90% yield, yellow powder). 4a 1 H NMR spectra, see figure 1 , 13 C NMR spectra see figure 2 .
[0042] 5,11-Dithia-benzo [a] fluorene-6-thione (4a)
[0043] 1 H NMR (500MHz, CDCL 3 ): Δ8.15 (dd, J 1 = 2.4Hz, J 2 = 6.6Hz, 1H), 8.05 (dd, J 1 = 1.5Hz, J 2 = 7.8Hz, 1H), 7.68 (dd, J 1 = 1...
Embodiment 2
[0046] 4b Preparation of the product: Example 2
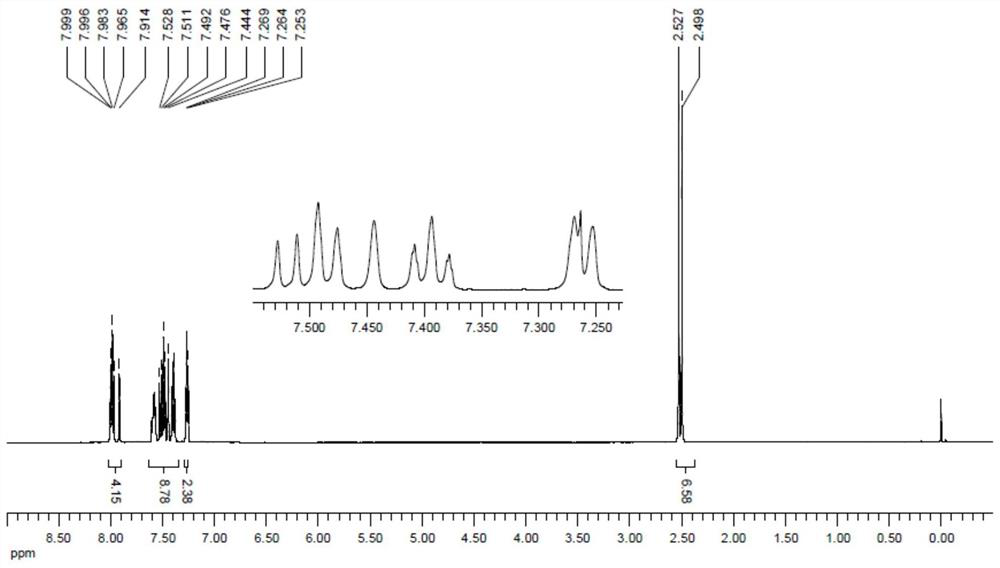
[0047] At room temperature, so a round bottom flask was added 25mL of 5mmol2- mercapto cinnamic acid, 6mmol 5mmol carbon disulfide and 2-bromo-5-methyl thiophenol in acetonitrile followed by addition of 15mL, 0.5mmol platinum chloride and 10mmol of sodium in ethanol, and the reaction was stirred at 80 ℃ 8 hours. After cooling, to the system was added 20mL saturated sodium chloride aqueous solution, and extracted 3 times with ethyl acetate, 20mL each, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was evaporated, 200-300 mesh silica gel column chromatography to give coumarin and the dithiophosphoric benzothiophene compound 4b (1356mg, 91% yield, yellow powder). 4b 1 H NMR spectra, see image 3 , 13 C NMR spectra see Figure 4 .
[0048] 9-Methyl-5,11-dithia-benzo [a] fluorene-6-thione (4b)
[0049] 1 H NMR (500MHz, CDCL 3 ): Δ7.98 (m, 3H), 7.91 (s, 1H), 7.55-7.61 (m, 2H), 7.52 (d, J = 8.5Hz, 1H), 7.49...
Embodiment 3
[0051] 4c Preparation of the product: Example 3
[0052] At room temperature, so a round bottom flask was added 25mL of 5mmol2- mercapto cinnamic acid, 6mmol 5mmol carbon disulfide and 2-bromo-4-methyl thiophenol in acetonitrile followed by addition of 15mL, 0.5mmol platinum chloride and 10mmol of sodium in ethanol, and the reaction was stirred at 80 ℃ 8 hours. After cooling, to the system was added 20mL saturated sodium chloride aqueous solution, and extracted 3 times with ethyl acetate, 20mL each, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was evaporated, 200-300 mesh silica gel column chromatography to give coumarin and the dithiophosphoric benzothiophene compound 4c (1222mg, 82% yield, yellow powder). 4c 1 H NMR spectra, see Figure 5 , 13 C NMR spectra see Image 6 .
[0053] 8-Methyl-5,11-dithia-benzo [a] fluorene-6-thione (4c)
[0054] 1 H NMR (500MHz, CDCL 3 ): Δ7.98 (m, 3H), 7.91 (s, 1H), 7.55-7.61 (m, 2H), 7.52 (d, J = 8.5Hz, 1H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com