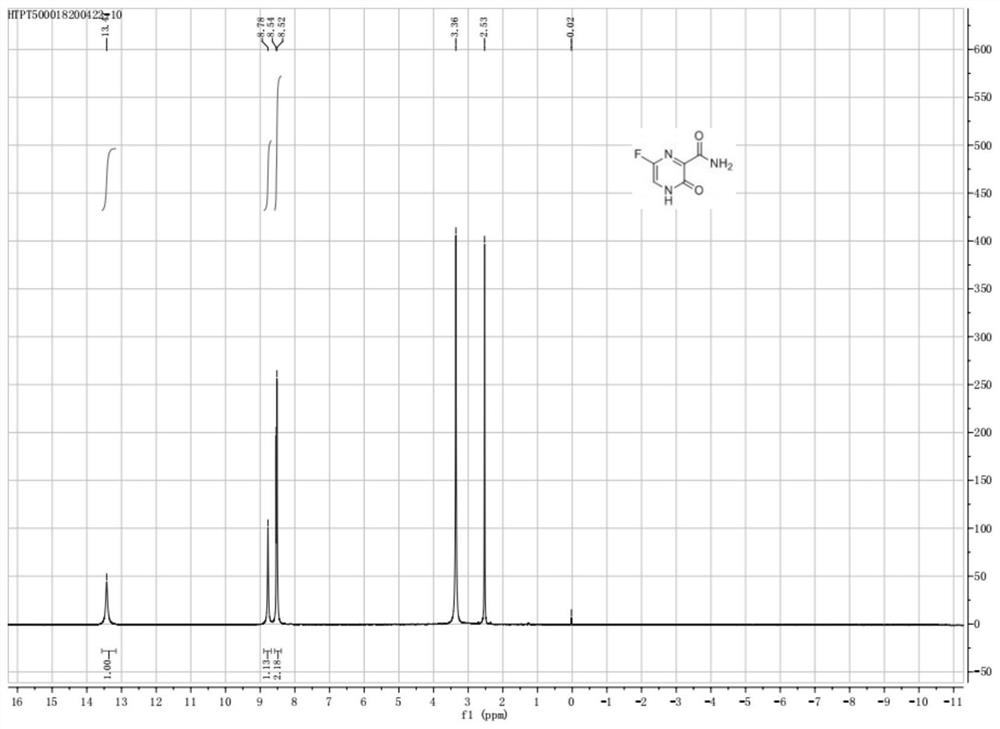
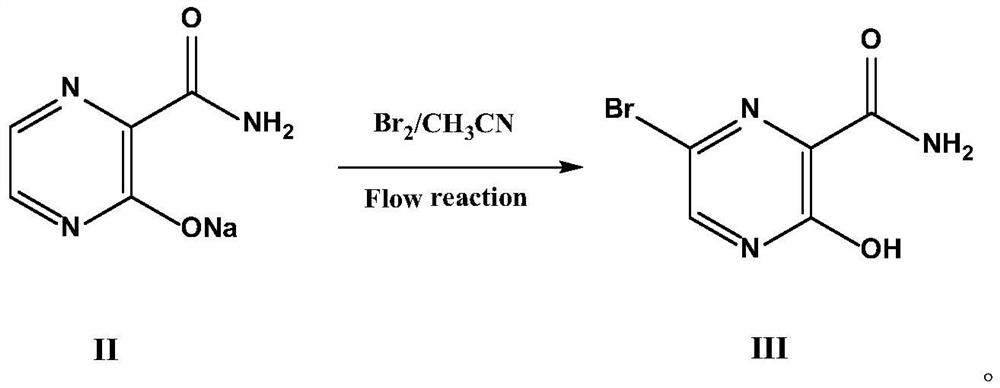
Fapiravir and preparation method of intermediate thereof
A technology of favipiravir and intermediates, which is applied in the field of preparation of favipiravir and its intermediates, can solve the problems of redness, swelling and itching at contact parts, increase the separation and purification process, and have potential safety hazards, and achieve low raw material prices , high reaction efficiency, high yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 compound II
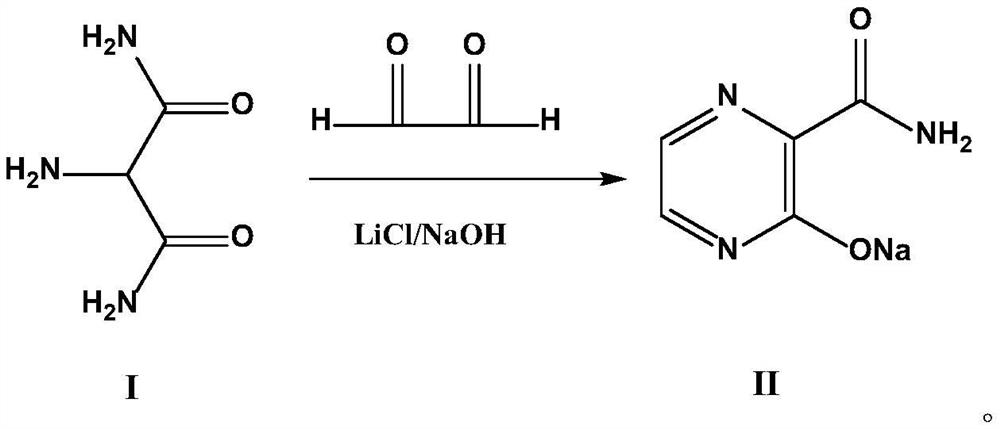
[0049] Into a 5L three-necked reaction flask, add 800g of 20% NaOH solution at 25°C, add 4.2g (0.1mol) of LiCl, add 1171.1g (10mol) of compound I aminomalonamide, stir evenly and start to add 40% glyoxal dropwise The solution was 1741.2g (12mol), and the dropwise addition was completed after 30 minutes. After the dropwise addition, the reaction was continued at 25°C for 3h. After the reaction was completed, it was filtered, and the filter cake was washed with 600mlx2 of 80% acetonitrile solution, and then dried in an oven at 80°C to obtain 1420.9g of yellow solid compound II (8.82mol), with a yield of 88.2%.
Embodiment 2
[0050] The preparation of embodiment 2 compound II
[0051] Into a 5L three-necked reaction flask, add 800g of 20% NaOH solution at 20°C, add 4.2g (0.1mol) of LiCl, add 1171.1g (10mol) of compound I aminomalonamide, stir well and start to add 40% glyoxal dropwise The solution was 1741.2g (15mol), and the dropwise addition was completed after 30 minutes. After the dropwise addition was completed, the reaction was continued at 20°C for 3h. After the reaction was completed, it was filtered, and the filter cake was washed with 600mlx2 of 80% acetonitrile solution, and then dried in an oven at 80°C to obtain 1438.6g of yellow solid compound II (8.93mol), with a yield of 89.3%.
Embodiment 3
[0052] The preparation of embodiment 3 compound II
[0053] Into a 5L three-necked reaction flask, add 800g of 20% NaOH solution at 40°C, add 4.2g (0.1mol) of LiCl, add 1171.1g (10mol) of compound I aminomalonamide, and start to add 40% glyoxal dropwise after stirring evenly The solution was 1741.2g (11mol), and the dropwise addition was completed after 30min, and the reaction was continued at 40°C for 3h after the dropwise addition. After the reaction was completed, it was filtered, and the filter cake was washed with 600mlx2 of 80% acetonitrile solution, and then dried in an oven at 80°C to obtain 1401.5g of yellow solid Compound II (8.7mol), with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com