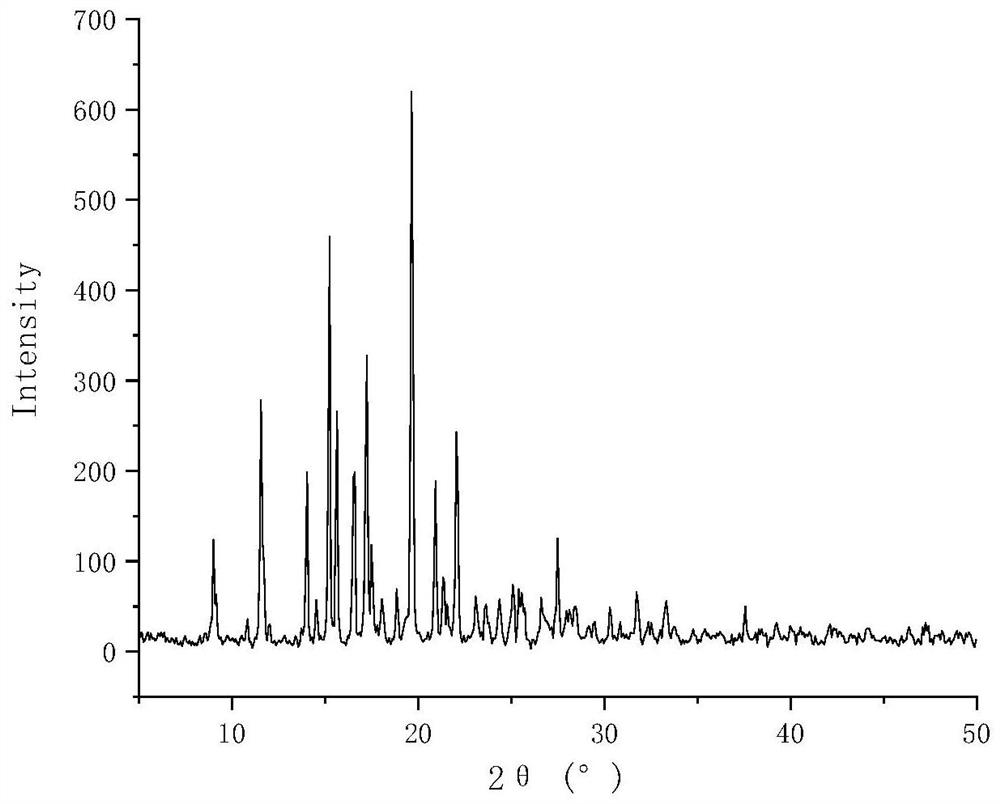
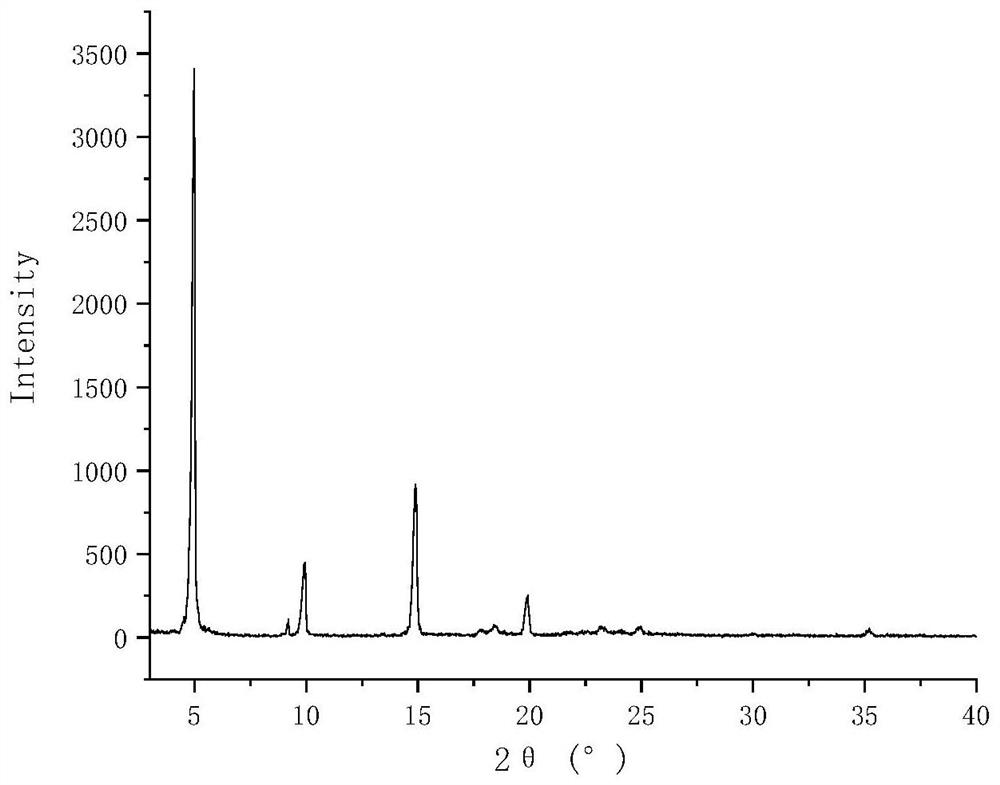
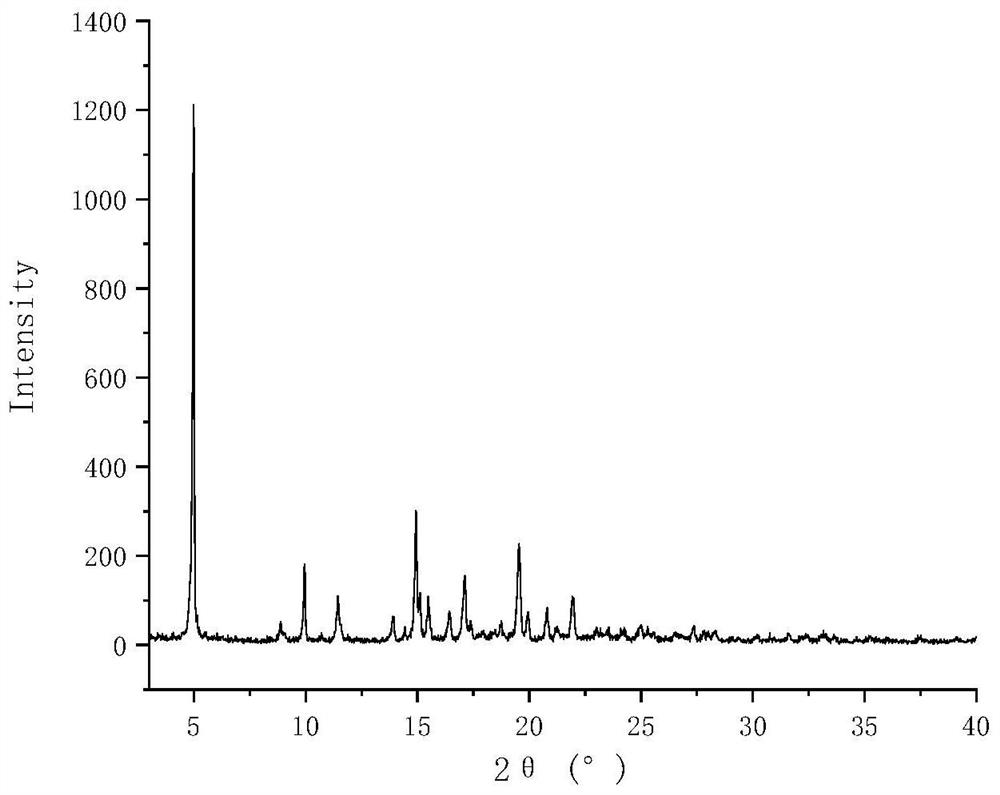
Lurasidone hydrochloride tryptophan coamorphous substance/lurasidone hydrochloride L-proline coamorphous substance and production method and application thereof
A technology for lurasidone hydrochloride tryptophan and lurasidone hydrochloride, which is applied in the field of medicine, can solve the problems of low solubility and slow dissolution rate, and achieves the effects of simple preparation, improved applicability, and improvement of clinical adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of lurasidone hydrochloride tryptophan co-amorphous
[0063] Weigh 0.5g of lurasidone hydrochloride and 0.193g of tryptophan into the same 100mL eggplant-shaped bottle, add 50mL of methanol, stir at room temperature and sonicate until completely dissolved to obtain a clear and transparent solution. The solution was placed on a vacuum rotary evaporator, and the temperature of the water bath was 55 °C. After the methanol solvent evaporated completely, the corresponding solid product was obtained. Place it in a vacuum drying oven at room temperature for 24 hours to remove the residual solvent in the rotary evaporated product, take out the final product in an environment with a relative humidity of less than 30% RH, and store it at 4°C with a humidity of less than 30% RH.
Embodiment 2
[0065] Preparation of lurasidone hydrochloride tryptophan co-amorphous
[0066] Weigh 0.5g of lurasidone hydrochloride and 0.193g of tryptophan into the same 100mL eggplant-shaped bottle, add 50mL of methanol, stir at room temperature and sonicate until completely dissolved to obtain a clear and transparent solution. The solution was placed on a vacuum rotary evaporator, and the temperature of the water bath was 35 °C. After the methanol solvent evaporated completely, the corresponding solid product was obtained. Place it in a vacuum drying oven at room temperature for 24 hours to remove the residual solvent in the rotary evaporated product, take out the final product in an environment with a relative humidity of less than 30% RH, and store it at 4°C with a humidity of less than 30% RH.
Embodiment 3
[0068] Preparation of lurasidone hydrochloride tryptophan co-amorphous
[0069] Weigh 0.5g of lurasidone hydrochloride and 0.193g of tryptophan into the same 100mL eggplant-shaped bottle, add 60mL of ethanol, stir at room temperature and sonicate until completely dissolved to obtain a clear and transparent solution. The solution was placed on a vacuum rotary evaporator, and the temperature of the water bath was 60 °C. After the ethanol solvent evaporated completely, the corresponding solid product was obtained. Place it in a vacuum drying oven at room temperature for 24 hours to remove the residual solvent in the rotary evaporated product, take out the final product in an environment with a relative humidity of less than 30% RH, and store it at 4°C with a humidity of less than 30% RH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com