Reagent composition for ph measurement
A technology of compositions and reagents, applied in the direction of testing pH value, using chemical indicators for analysis, and material analysis by observing the effect on chemical indicators, which can solve problems such as stenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach example
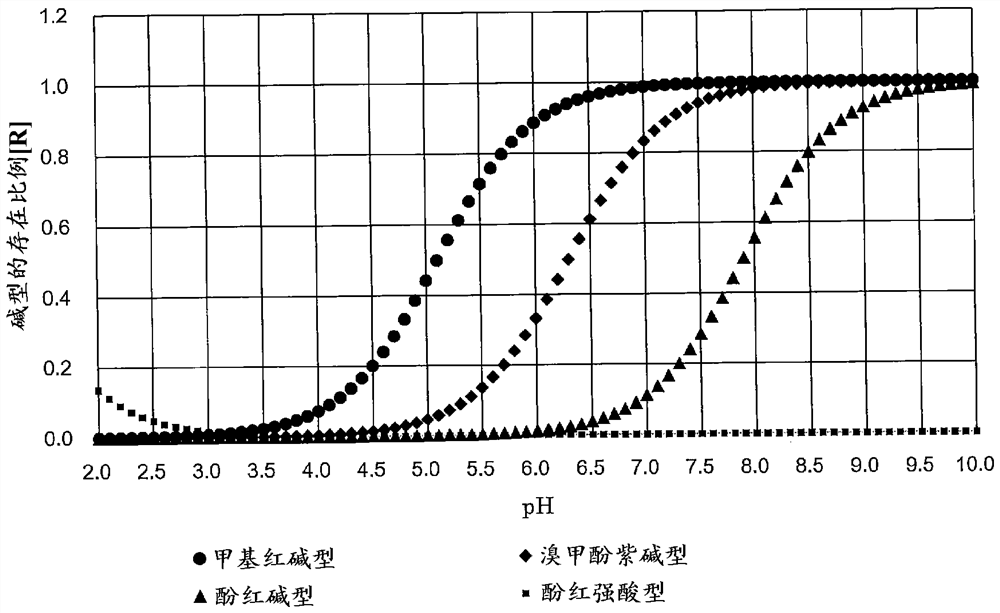
[0037] This embodiment example assumes that the pH of the test water is measured in the range of approximately 4 to 9 (this range includes the entire buffer pH range of carbonic acid), and contains the following color reagents.
[0038] ◎The first chromogenic reagent
[0039] Selected from chromogenic reagents with a pKa in the range of 4.1 to 6.0. For example, it may be selected from methyl red (pKa: 5.1), bromophenol blue (pKa: 4.2) and bromocresol green (pKa: 4.7).
[0040] ◎The second chromogenic reagent
[0041] Selected from chromogenic reagents with a pKa in the range of 6.5 to 8.5. For example, it may be selected from phenol red (pKa: 1.2 and 7.7), neutral red (pKa: 6.7 and 7.4) and cresyl red (pKa: 1.0 and 8.0).
[0042] ◎The third chromogenic reagent
[0043]Selected from chromogenic reagents with a pKa in the range of 5.5 to 7.5. For example, it may be selected from bromocresol purple (pKa: 6.3) and bromothymol blue (pKa: 7.1).
[0044] Specific examples of th...
no. 2 Embodiment approach example
[0060] This embodiment example assumes that the pH of the test water is measured in the range of approximately 4 to 12 (this range also includes the entire buffer pH range of carbonic acid), and contains the following color reagents.
[0061] ◎The first chromogenic reagent
[0062] Selected from chromogenic reagents with a pKa in the range of 4.1 to 6.0. For example, it may be selected from methyl red (pKa: 5.1), bromophenol blue (pKa: 4.2) and bromocresol green (pKa: 4.7).
[0063] ◎The second chromogenic reagent
[0064] Selected from chromogenic reagents with a pKa in the range of 8.5 to 11.5. For example, it may be selected from Alizarin Yellow (pKa: 11.06) and Thymol Blue (pKa: 1.7 and 8.9).
[0065] ◎The third chromogenic reagent
[0066] Two kinds of color-developing reagent A selected from the color-developing reagents whose pKa is in the range of 5.5 to 7.5, and color-developing reagent B selected from the color-developing reagents whose pKa is in the range of 7.0...
Embodiment
[0135] 500 g of reagent compositions having the compositions shown in Table 5 were prepared. This reagent composition corresponds to the reagent composition listed as a specific example of the reagent composition of the first embodiment.
[0136] [table 5]
[0137] table 5
[0138]
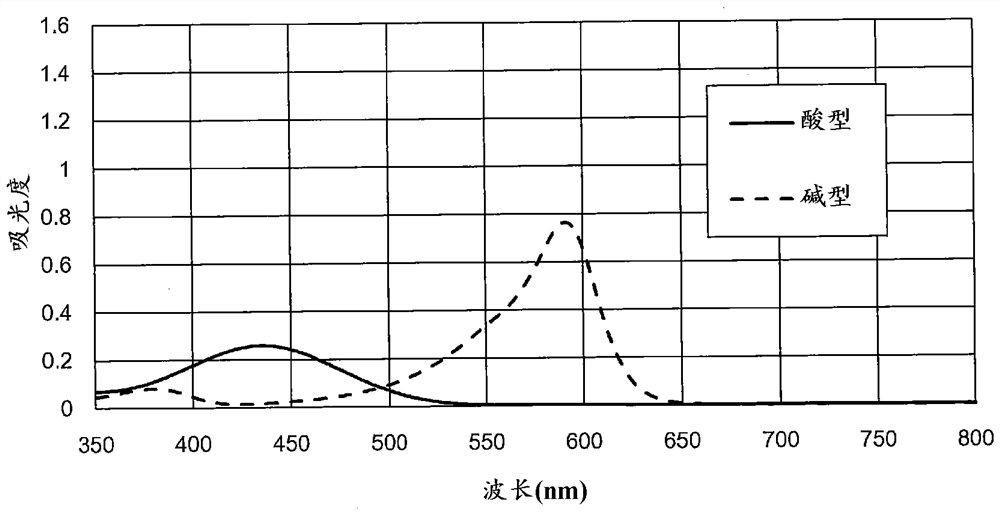
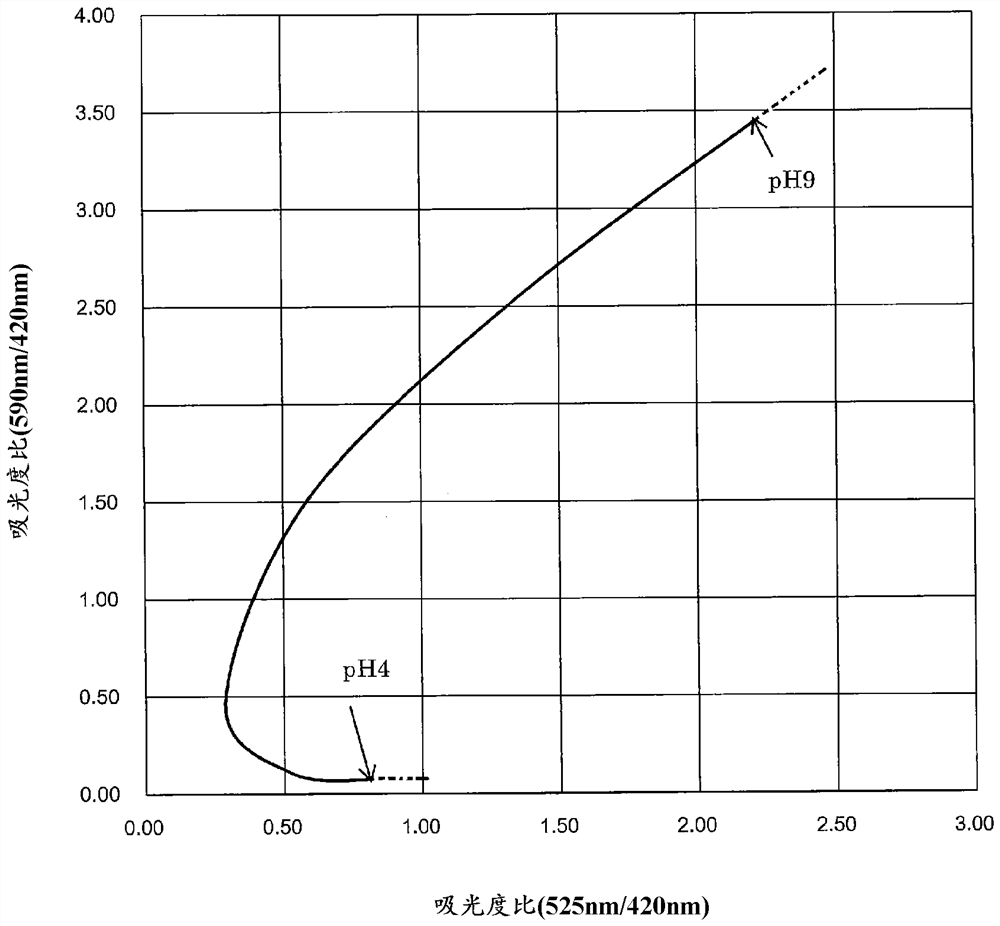
[0139] Assuming that 100mL test water added with 0.75g of reagent composition is irradiated with visible light with wavelengths of 420nm, 525nm and 590nm, according to the above formula (1), formula (2) and formula (3) and Henderson-Hassell Balcher's formula calculates the absorbance of visible light at each wavelength predicted in this case. Here, the absorbance of the above-mentioned visible light of each wavelength is calculated for the test water whose pH is different in the range of 1 to 10 at intervals of 0.1.
[0140] According to the calculated absorbance of each wavelength, the relationship between the pH value of the test water and the absorbance ratio (525nm / 420nm) and the relatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com