Application of a kind of indanone derivative in preparation of medicine for treating sepsis
A kind of technology of sepsis and derivatives, applied in the application field of indanone derivatives in the preparation of drugs for treating sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
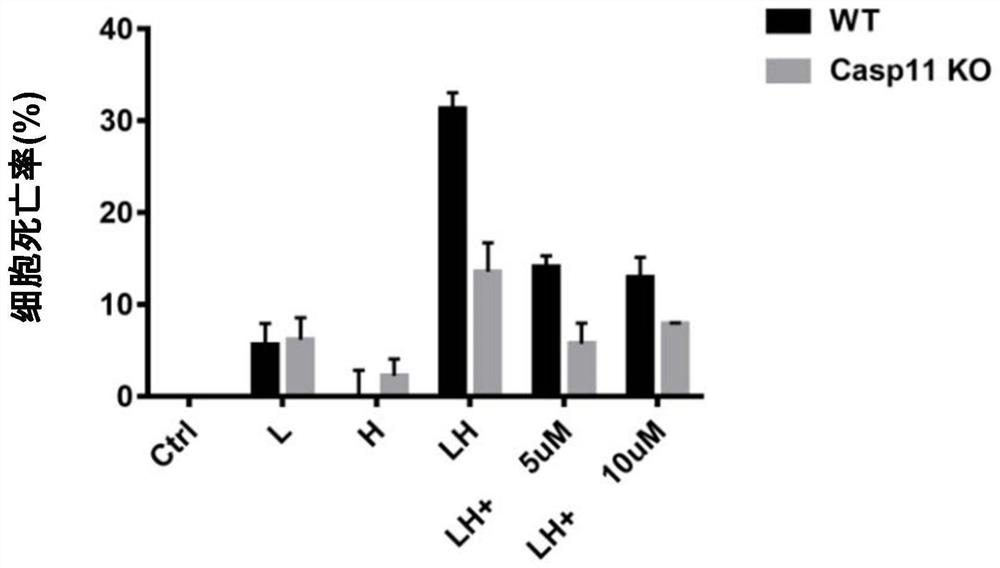
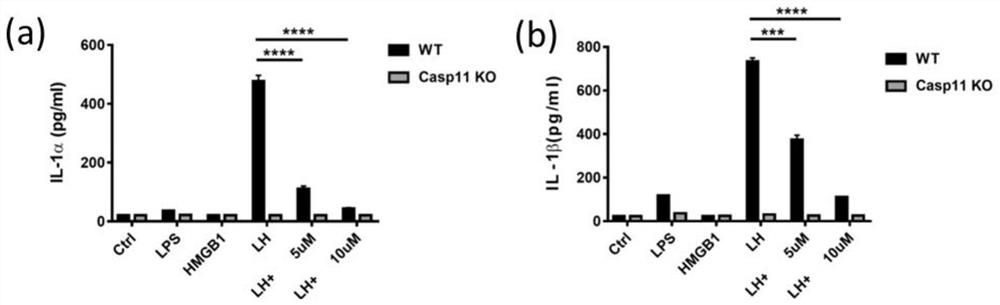
[0050] Example 1: Compounds inhibit primary macrophage pyroptosis induced by LH (LPS+HMGB1) activation of Caspase11
[0051] High-mobility group box 1 protein (HMGB1) is a kind of protein that is highly conserved and widely expressed in mammals. It mainly exists in the nucleus under normal conditions. The medium is released to the outside of the cell and plays an important pathogenic role in diseases or tissue damage (Regulation of post-translational modifications of HMGB1 during immune responses. Antioxid Redox Signal. 2016 Apr 20; 24(12): 620-34.). Invention It was found that HMGB1 released by hepatocytes in sepsis can transport circulating LPS to the cytoplasm of vascular endothelial cells and macrophages, and initiate Caspase-11-mediated pyroptosis (The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-DependentLethality in Sepsis.Immunity.2018 Oct 16; 49(4):740-753.). During the above process, liver cells recognize circulating LPS through their own expressed TLR4 recep...
Embodiment 2
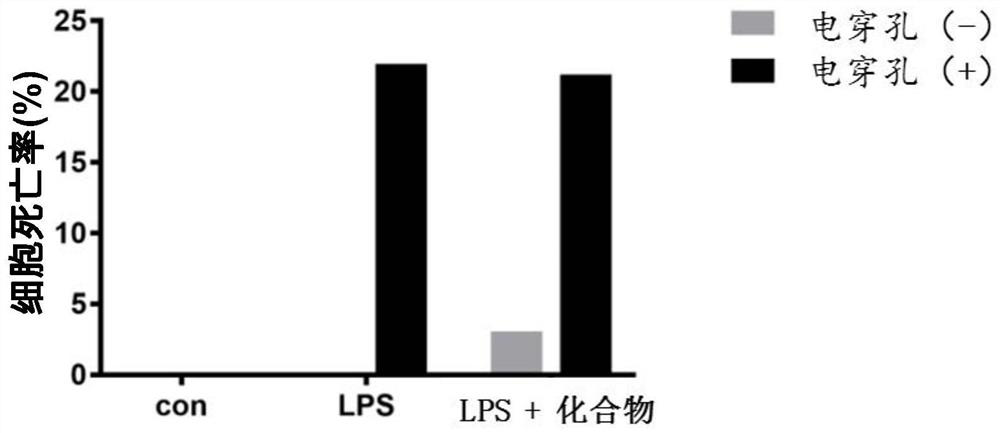
[0063] Example 2: Compounds inhibit LPS entry into cells to inhibit Caspase11-dependent pyroptosis
[0064] 1. Experimental steps: Extract the primary peritoneal macrophages of WT mice, resuspend the cells in RPMI-1640 complete medium to adjust the concentration and plant them in a 6-well plate, 2×10^6 cells / well, wash the cells twice with DBPS after adhering to the wall overnight Change to 1640 serum-free medium. Compound 10 μM final concentration was added to the cells for pre-incubation for 1 h, then the mixture of LPS 1 μg / ml+HMGB 1400 ng / ml (pre-incubated at room temperature for 20 min) was added to stimulate the cells for 2 h, after the cells were washed 3 times with DPBS, the cells were digested with 300 μl / well of 0.25% trypsin, 400μl / well 1640 complete medium to stop the digestion, gently pipet the cells into a 1.5ml sterile enzyme-free EP tube, wash the cells 3 times with DPBS, add 0.005% digitonin (digitonin) 200μl / EP tube and lyse on ice for 10-15min , 13000rpm, c...
Embodiment 3
[0066] Example 3: Protective effect of compounds on mice with endotoxemia
[0067] 1. Experimental procedure: select mice weighing 25-30 g, and divide them into LPS+DMSO group, LPS+compound group and DMSO complete control group. The compound was dissolved in DMSO, and the concentration of the stock solution was 40 mg / ml. The compound was dosed at 4 mg / kg, 100 μl per mouse, injected intraperitoneally 1 hour in advance, and then injected with LPS 10 mg / kg, 100 μl per mouse; in the LPS+DMSO group, DMSO 100 μl was injected intraperitoneally 1 hour in advance, and then injected LPS 10 mg / kg, 100 μl per mouse. DMSO complete control group was injected intraperitoneally with DMSO 100μl / rat. The survival rate of mice in each group was observed within 5 days.
[0068] 2. Experimental results: the experimental results of this embodiment are as follows Figure 5 shown, from Figure 5 It can be seen that after using the compound, the survival rate of endotoxemia mice is improved, which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com