A kind of tricarboxylic amphoteric surfactant and preparation method thereof
A technology of surfactants and tricarboxylic groups, which is applied in the field of tricarboxylic amphoteric surfactants and its preparation, can solve the problems of high prices of surfactants, and achieve the effect of low cost and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
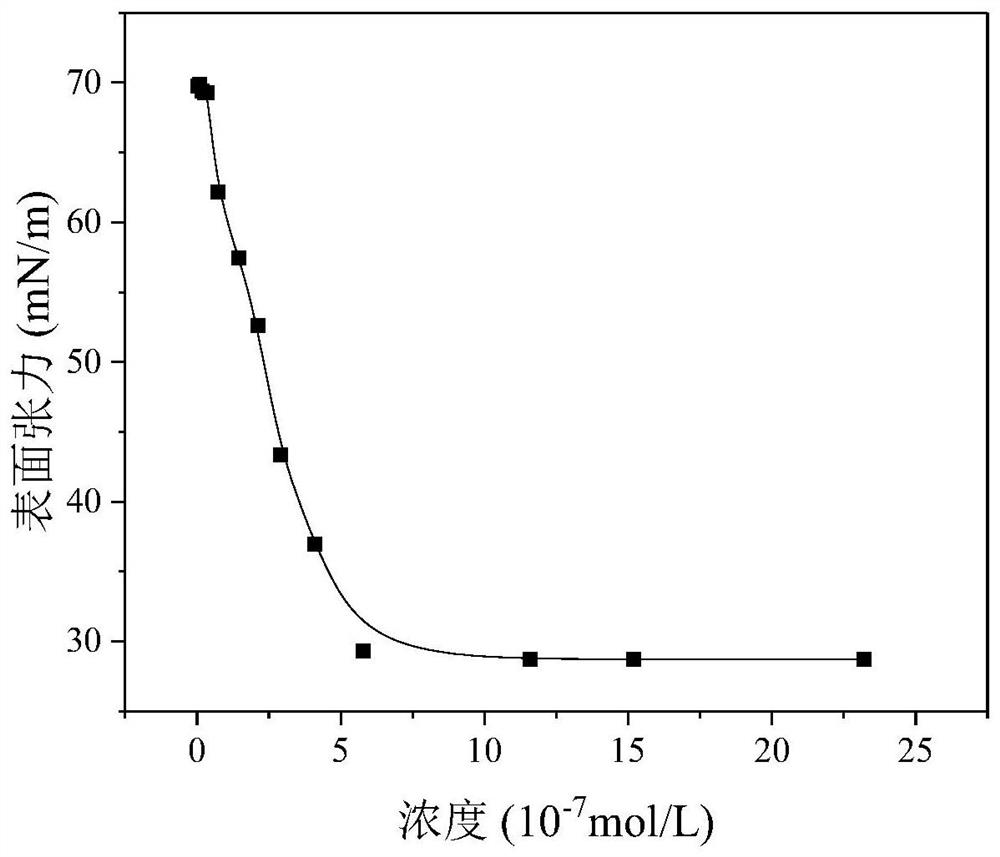
Examples
Embodiment 1
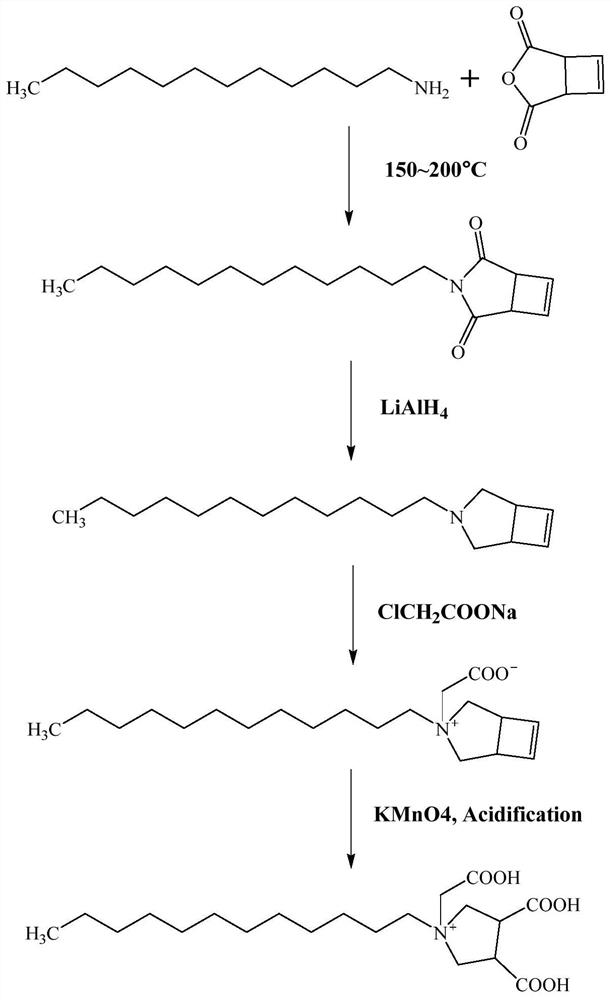
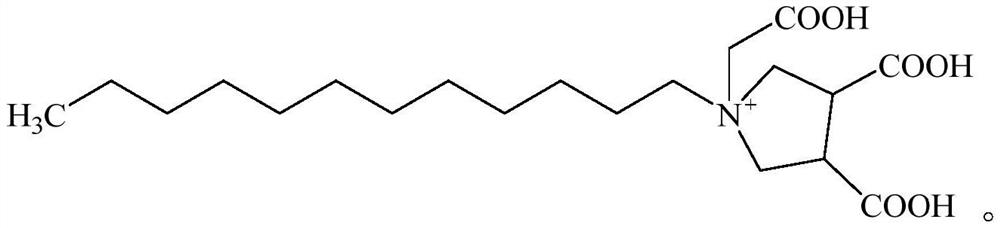
[0032] (1) Add dodecylamine (18.5g, 0.1mol) and cyclobut-3-ene dicarboxylic anhydride (24.8g, 0.2mol) dropwise successively in a 250mL three-necked flask equipped with a reflux condenser, nitrogen gas 3 times, Then heated to 180° C. for 3 h, and TLC (Thin Layer Chromatography, thin layer chromatography) monitored the completion of the reaction. After the reaction was completed, it was cooled to room temperature, 50 mL of distilled water was added, extracted three times with 80 mL of ethyl acetate, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to obtain a crude product, and finally separated by a column to obtain a colorless oily imide.
[0033] (2) Add 80 mL of tetrahydrofuran dried with sodium metal into a 250 mL three-neck flask, cool to 0°C in an ice-salt bath, and slowly add 3.8 g of LiAlH 4 After stirring for 3 minutes, the imide dissolved in tetrahydrofuran was slowly added dropwise into the three-necked flask, and after the additi...
Embodiment 2
[0037] (1) Add dodecylamine (18.5g, 0.1mol) and cyclobut-3-ene dicarboxylic anhydride (24.8g, 0.2mol) dropwise successively in a 250mL three-necked flask equipped with a reflux condenser, nitrogen gas 3 times, Then heated to 150° C. for 2 h, and TLC (Thin Layer Chromatography, thin layer chromatography) monitored the completion of the reaction. After the reaction was completed, it was cooled to room temperature, 50 mL of distilled water was added, extracted three times with 80 mL of ethyl acetate, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to obtain a crude product, and finally separated by a column to obtain a colorless oily imide.
[0038] (2) Add 90 mL of tetrahydrofuran dried with sodium metal into a 250 mL three-neck flask, cool to 0°C in an ice-salt bath, and slowly add 4.18 g of LiAlH in batches 4 After stirring for 3 minutes, the imide dissolved in tetrahydrofuran was slowly added dropwise into the three-necked flask, and afte...
Embodiment 3
[0042] (1) Add dodecylamine (18.5g, 0.1mol) and cyclobut-3-ene dicarboxylic anhydride (24.8g, 0.2mol) dropwise successively in a 250mL three-necked flask equipped with a reflux condenser, nitrogen gas 3 times, Then heated to 200° C. for 5 h, and TLC (Thin Layer Chromatography, thin layer chromatography) monitored the completion of the reaction. After the reaction was completed, it was cooled to room temperature, 50 mL of distilled water was added, extracted three times with 80 mL of ethyl acetate, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to obtain a crude product, and finally separated by a column to obtain a colorless oily imide.
[0043] (2) Add 100 mL of tetrahydrofuran dried with sodium metal into a 250 mL three-neck flask, cool to 0°C in an ice-salt bath, and slowly add 3.99 g of LiAlH 4 After stirring for 3 minutes, the imide dissolved in tetrahydrofuran was slowly added dropwise into the three-necked flask, and after the addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com