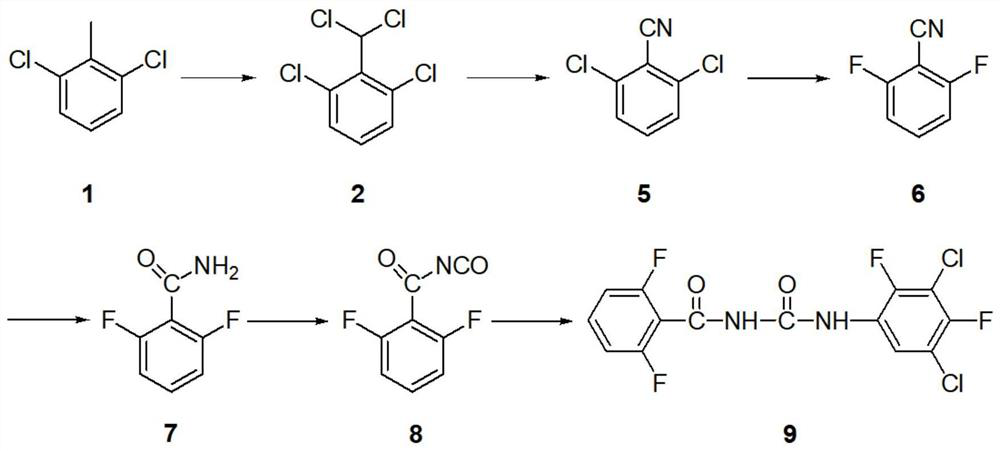
Synthesis method of insecticide teflubenzuron and intermediate 2,6-difluorobenzamide of insecticide teflubenzuron
A technology of difluorobenzamide and difluorobenzoyl isocyanate is applied in the field of synthesis of insecticide fuzuron and its intermediate 2,6-difluorobenzamide, which can solve the pressure of environmental protection and the process route It can improve the yield and quality, shorten the reaction route and reduce the pressure of environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: The synthesis of compound 2→5 was completed by "one-pot method".
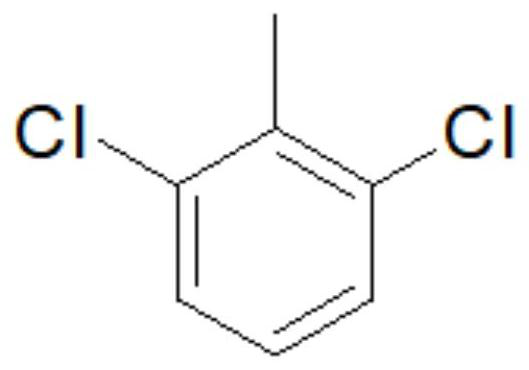
[0057] Set the reaction temperature to 150°C in the CORNING G1-10FM SiC high-flux microchannel reactor, pump the suspension prepared by stirring 1610g2,6 dichlorotoluene and 15g phosphorus pentachloride at a flow rate of 20mL / min, 40g / Chlorine gas is introduced at a flow rate of min, the material is introduced into the gas-liquid separator filled with water, and the tail gas is passed into the tail gas absorption device. Open the gas-liquid separator bottom valve, separate layers, the water layer is reclaimed hydrochloric acid, as a by-product, and the organic layer is crude product. The main fraction was collected by rectification of the crude product to obtain 222 g of 2,6-dichlorobenzylidene dichloride, with a content of 99% and a yield of 97.0%;
[0058] 57.5 g of 2,6-dichlorobenzylidene dichloride, 300 g of 85% acetic acid, 0.5 g of zinc chloride, 21 g of hydroxylamine hydrochloride and...
Embodiment 4
[0081]
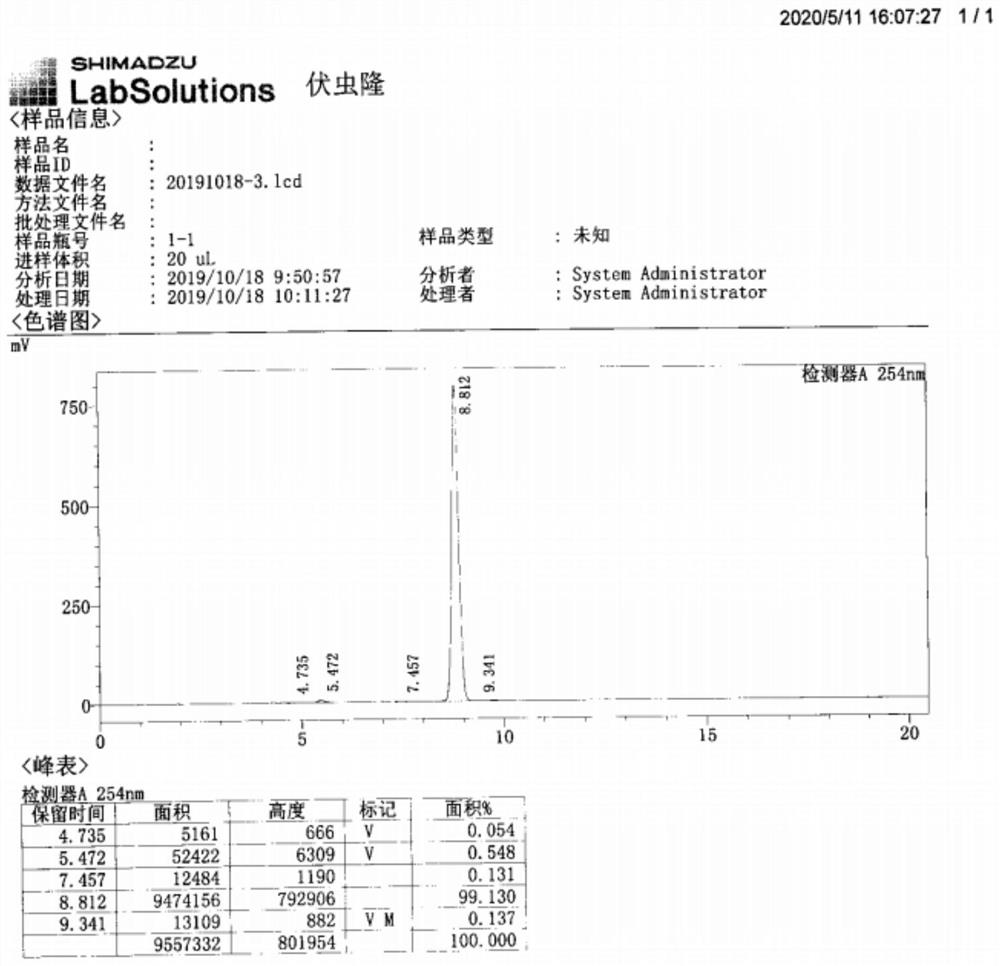
[0082] Table (one): content and yield result table of embodiment one compound 2, 5, 6, 7, 8 and 9
[0083]
[0084] Table (two): content and yield result table of embodiment two compounds 2, 5, 6, 7, 8 and 9
[0085]
[0086] Table (three): content and yield result table of embodiment three compounds 2, 5, 6, 7, 8 and 9
[0087] By comparing and analyzing the above three tables, we found the following results:
[0088] 1. The total yield of Example 1 is about 67.7%, and the total yield of Example 2 is about 70.4%, which is greatly improved compared with the existing 55.4%. Compound 2→5 adopts "one pot method" After completion, the yield of Compound 5 in Example 1 was 94%, with a content of 99.7%, and the yield of Compound 5 in Example 2 was 93.6%, with a content of 99.5%. The yields were all above 93%, and the content was higher than 99.5%.
[0089]2, embodiment one, embodiment two and embodiment three all adopt microchannel chlorination reaction to synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com