Compounds and their uses
A technology for compounds and uses, applied in the field of compounds, can solve the problems of inconvenient storage and transportation, unstable activity, short half-life, etc., and achieve the effects of convenient treatment plan, short preparation period and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 compound synthesis
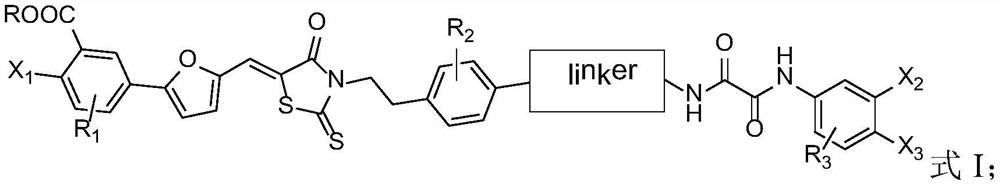
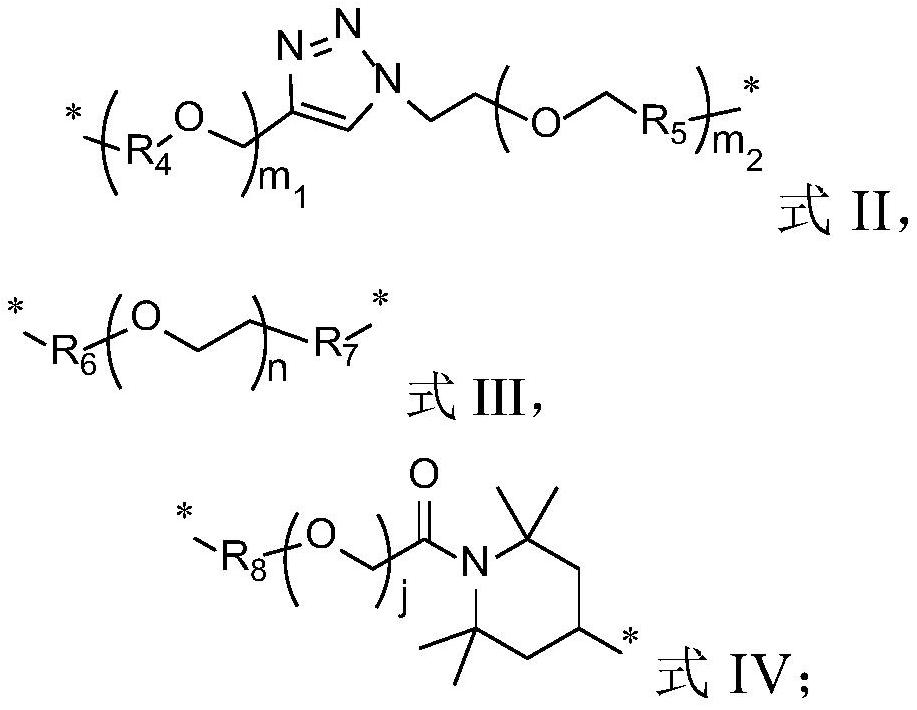
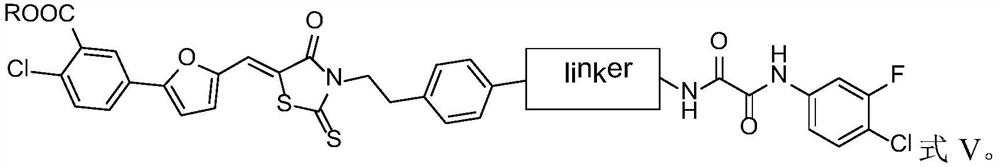
[0071] Exemplarily, in this example, a compound with the structure shown in the following formula was synthesized, wherein the structure shown in the linker, the substituent R and the compound number are listed in Table 1:
[0072]
[0073] Table 1 Compound numbers and corresponding substituents
[0074]
[0075] (1) Synthesis of Compounds 1a-1e
[0076] 1) Synthetic method of compound 18a-b
[0077]
[0078] Reagents and conditions: (i) Boc-Tyramine, K2CO3, DMF, 80°C, 12h; (c) AcCl, CH3OH, 0°C-room temperature, 24h; (ii) bis(carboxymethyl)trithiocarbonate, Et3N, DME, MW, 90 ℃, 15min.
[0079] 1. Compound 17a
[0080] Potassium carbonate (2.4g, 17.5mmol) and 80% propyne bromide (16a 2.0mL, 17mmol) were successively added to a 30mL DMF solution of Boc-tyramide (3.5g, 14.7mmol), heated to 80°C for 12h, and stopped Heating, after the reaction was cooled to room temperature, extracted with ethyl acetate, washed with water, washed...
Embodiment 2
[0184] Example 2 Compound Structural Characterization
[0185] The structures of the compounds were characterized by NMR and mass spectrometry.
[0186] Compound 1a: 56 mg of orange solid product was prepared from S1a (50 mg, 95.4 μmol) and S2a (30 mg, 105 μmol), with a yield of 72%.
[0187] 1 H NMR (400MHz, DMSO-d 6 )δ13.71(br s,1H),11.02(s,1H),9.26(t,J=6.0Hz,1H),8.22(s,1H),7.93(dd,J=11.2,2.0Hz,2H) ,7.74-7.66(m,3H),7.59(t,J=8.8Hz,1H),7.48(d,J=4.0Hz,1H),7.40-7.37(m,1H),7.14(d,J=8.4 Hz, 2H), 6.96(d, J=8.4Hz, 2H), 5.08(s, 2H), 4.57(t, J=5.6Hz, 2H), 4.19(t, J=7.6Hz, 2H), 3.70- 3.66(m,2H,),2.87(t,J=7.6Hz,2H); 13 C NMR (100MHz, DMSO-d 6 )δ193.54,166.27,159.67,158.45,157.94,156.77,155.51,149.70,137.18,138.08,130.49,129.81,129.65,124.75,122.92,119.15,118.09,117.32,117.29,114.69,114.44,111.48,108.60,108.34,61.04 , 48.17, 45.40, 31.24; ESI-MS (m / z) 809 [M-H] - .
[0188] Compound 1b: 60 mg of red solid product was obtained from S1b (60 mg, 105 μmol) and S2a (33 mg, 116 μmol),...
Embodiment 3
[0206] The inhibitory effect of each compound of embodiment 3 to laboratory adaptation strain HIV-1IIIB
[0207] According to literature (Xu, W.; Pu, J.; Su, S.; Hua, C.; Su, X.; Wang, Q.; Jiang, S.; Lu, L., Revisiting the mechanism of enfuvirtide and designing An analog with improvedfusion inhibitory activity by targeting triple sites in gp41.Aids 2019, 33, 1545-1555) was used to detect the inhibitory effect of the compound prepared in Example 1 on the replication of laboratory-adapted strain HIV-1IIIB.
[0208] In the examples of this application, the HIV-1IIIB strain is provided by the AIDS Reagent and Reference Project of the US NIH, catalog number 398.
[0209] HIV-1IIIB (X4) was amplified on MT-2 cells (provided by the AIDS Reagent and Reference Project of the US NIH, catalog number 237) and its titer was determined, and the aliquots were stored at -80°C. To determine the antiviral activity of the compounds, each compound was dissolved in DMSO, and the initial concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com