Light-operated cell-free protein synthesis method, plasmid used in method and product using method
A protein, cell-free technology, applied in animal/human proteins, medical preparations containing active ingredients, introduction of foreign genetic material using carriers, etc., can solve difficult to achieve precise positioning of targeted cells, adverse effects of chemical stability problems, to achieve rapid response, increased flexibility, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1: Making pDark plasmid, culturing it in the cell-free extract of E.coli BL21, and expressing red fluorescent protein under dark and blue light irradiation conditions
[0133] - Make pDark plasmid
[0134] Use the PCR method to clone the red fluorescent protein gene mCherry from the plasmid pET23a-mCherry (the plasmid was synthesized by Jinweizhi Company), and then use the PCR technology to clone the plasmid pDusk (the product number of the plasmid is: Addgene #43795) from the promoter FixK2 To ensure that there are 25 bases of the same sequence at both ends of the mCherry gene and the linear vector. The mCherry gene was connected with the vector by Gibson's self-assembly method to form the pDark plasmid.
[0135] - Prepare E.coli BL21 cell extract and mix with pDark to form a cell-free reaction system
[0136] The cells were cultured to prepare cell extracts E.coli BL21(DE3) containing two light-regulated proteins, and the cell-free system was prepared accord...
Embodiment 2
[0141] Example 2: Making pLight plasmid, culturing it in the cell-free extract of E.coli BL21, and expressing red fluorescent protein under dark and blue light irradiation conditions
[0142] -The pLight plasmid was further constructed on the basis of the pDark plasmid structure. pLight was based on the pDark, and the cI-pR gene circuit was assembled behind the promoter FixK2. Open the empty vector pDawn (product number of the plasmid: Addgene #43796) from the promoter pR by using PCR technology to ensure that there are 25 bases of the same sequence at both ends of the mCherry gene and the linear vector. Use Gibson self-assembly method to connect mCherry gene and vector to form pLight plasmid. - Prepare E.coli BL21 cell extract and mix with pLight to form a cell-free reaction system
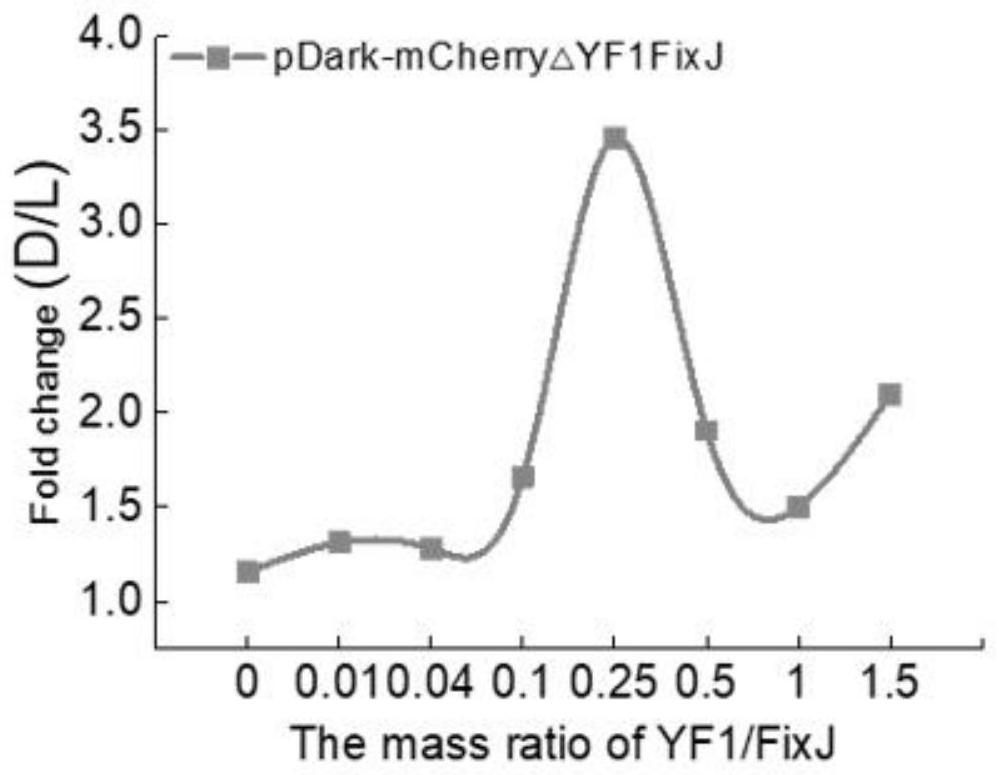
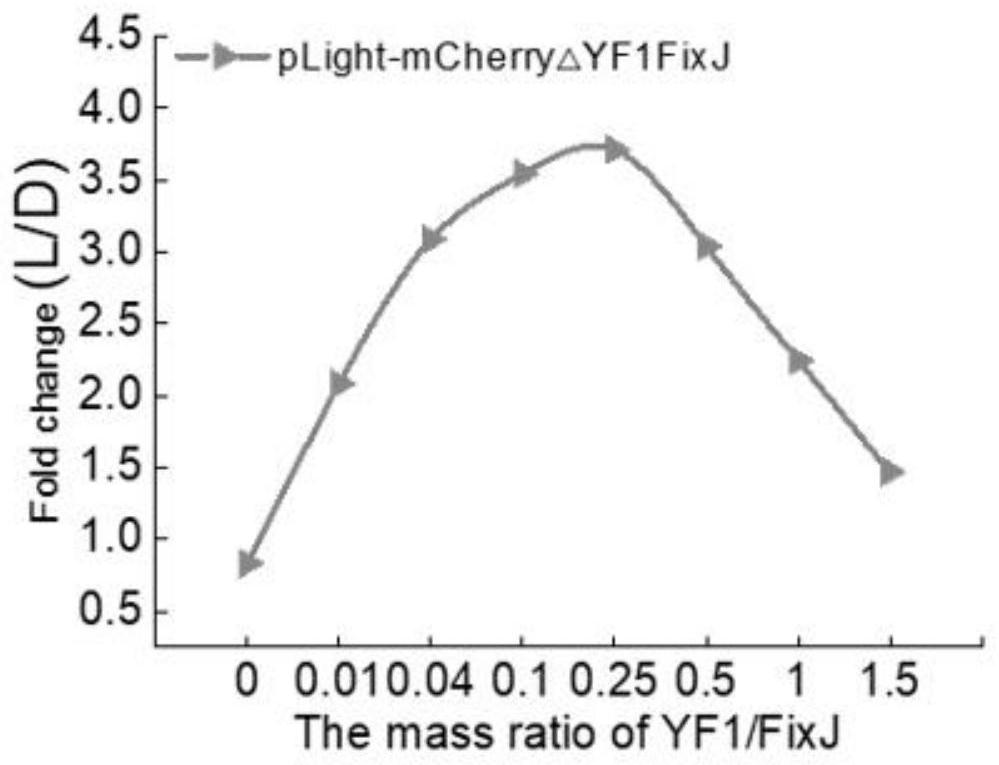
[0143] Prepare cell extracts E.coli BL21(DE3) containing two light-regulated proteins respectively. When preparing a cell-free system, mix the two cell extracts according to 0, 0.01, 0.04, 0.1, ...
Embodiment 3
[0148] Example 3: Select new pLight plasmids (replacing the red fluorescent protein with NbmCherry, NbRota3B2 and NbCEA5 three drug proteins), and study their expression in the cell-free extract of E.coli BL21.
[0149] - On the basis of the pLight plasmid structure in Example 2, pLight plasmids of three drug proteins were further constructed, namely pLight-NbmCherry, pLight-NbRota3B2 and pLight-NbCEA5. Use PCR technology to NbmCherry, the gene of NbRota3B2 and NbCEA5 three kinds of drug proteins from respective plasmid (can refer to following literature about NbmCherry: Fridy, P.C., Li, Y., Keegan, S., Thompson, M.K., Nudelman, I. , Scheid, J.F., Oeffinger, M., Nussenzweig, M.C., D., and Chait, B.T. (2014) A robust pipeline for rapid production of versatile nanobody repertoires, Nature methods 11, 1253. Regarding NbRota3B2, you can refer to the following literature: Vega, C.G., Bok, M., Vlasova, A.N., Chattha, K.S. ,Gómez-Sebastián,S., C., Alvarado, C., Lasa, R., Escriban...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com