1, 3-deuterated dansyl chloride and preparation method and application thereof
A technology of dansyl chloride and deuterium, applied in the field of isotope derivatization reagents, can solve problems such as differences in retention time and large difference in mass number of dansyl chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Prepare the reaction raw materials according to the following dosage ratio:
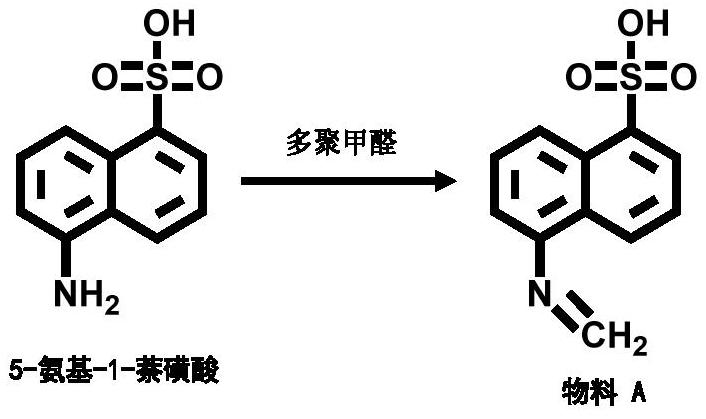
[0062] The mol ratio of paraformaldehyde and 5-amino-1-naphthalenesulfonic acid is 4.9:1;
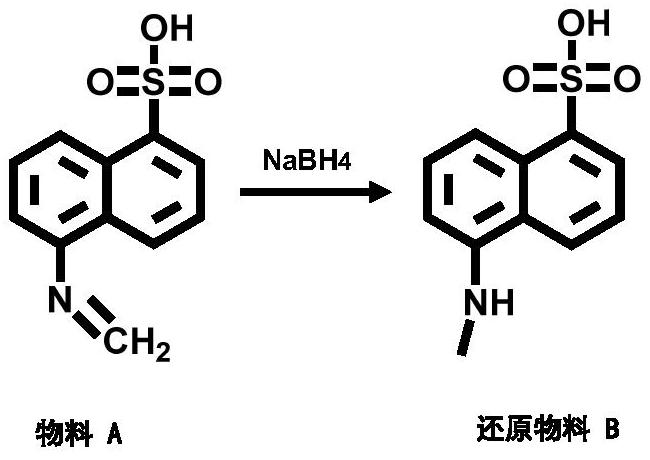
[0063] The mol ratio of sodium borohydride and 5-amino-1-naphthalenesulfonic acid is 2.0:1;
[0064] The mol ratio of deuteroiodomethane and reducing material B is 5.2:1;
[0065] The mol ratio of phosphorus oxychloride, phosphorus pentachloride and deuterium solid material is 9.1:2.2:1, and wherein, the amount of each component material is calculated with the mark molecular weight of commercially available product as the criterion, and the relative molecular weight of each component or The density parameters are listed in the brackets of the following steps, and the specific preparation process is:
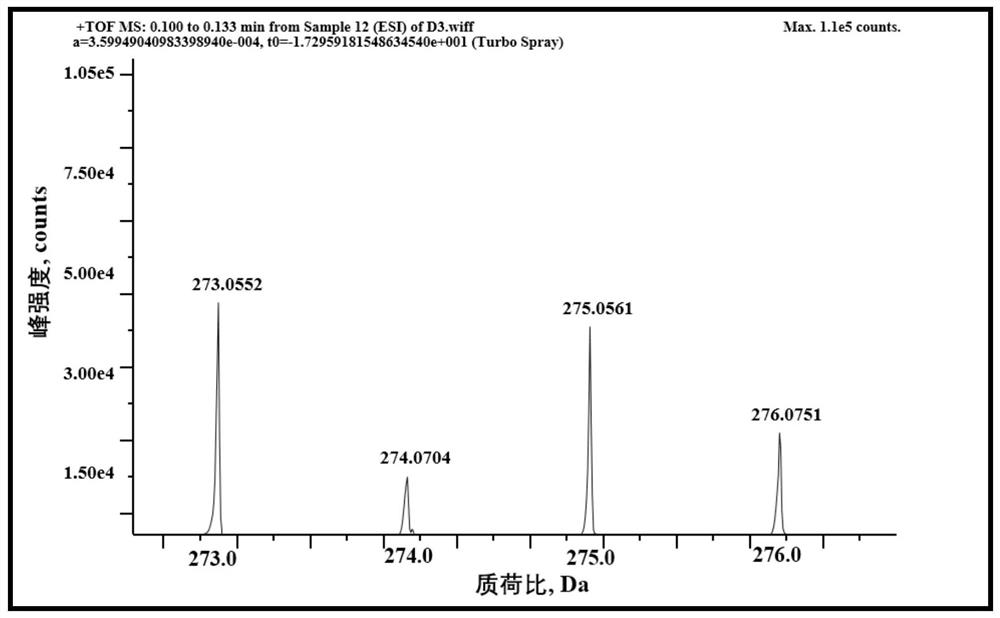
[0066] Step 1: Weigh 1.5g (223.25 / 0.0067mol) of 5-amino-1-naphthalenesulfonic acid into 30mL of methanol, and then add 2.17g (54.0 / 0.0434mol) of sodium methoxide. When the solution is brownish red, add ...
Embodiment 2
[0080] Prepare 3-deuterated dansyl chloride in the manner of Example 1, except that:
[0081] The mol ratio of paraformaldehyde and 5-amino-1-naphthalenesulfonic acid is 4:1;
[0082] The mol ratio of sodium borohydride and 5-amino-1-naphthalenesulfonic acid is 2:1;
[0083] The mol ratio of deuteroiodomethane and reducing material B is 4:1;
[0084]The mol ratio of phosphorus oxychloride, phosphorus pentachloride and deuterated solid material is 7:3:1;
[0085] The temperature of the first substitution reaction is 37° C., and the reaction time is 1.5 h; the yield of the obtained target product is 75%, and the purity is 80%.
Embodiment 3
[0087] Prepare 3-deuterated dansyl chloride in the manner of Example 1, except that:
[0088] The mol ratio of paraformaldehyde and 5-amino-1-naphthalenesulfonic acid is 3.5:1;
[0089] The mol ratio of sodium borohydride and 5-amino-1-naphthalenesulfonic acid is 2.7:1;
[0090] The mol ratio of deuterated methyl iodide and reducing material B is 4.2:1;
[0091] The mol ratio of phosphorus oxychloride, phosphorus pentachloride and deuterated solid material is 8:1.5:1;
[0092] The temperature of the first substitution reaction was 30° C., and the time was 2.5 h; the yield of the obtained target product was 78%, and the purity was 63%.
[0093] As can be seen from the above examples, the method provided by the present invention can prepare 3-deuterated dansyl chloride, the reaction raw materials are easy to obtain, the preparation method is simple and easy to control, and the yield is high, reaching more than 75%; the overall reliability of the preparation method is high, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com