A novel coronavirus S-protein polymer nanovaccine based on Helicobacter pylori ferritin
A technology of Helicobacter pylori and coronavirus, applied to viruses/bacteriophages, polypeptides containing positioning/targeting motifs, viruses, etc., can solve the problems of lack of vaccines, social burden crisis, lack of specific antiviral drugs, etc., and achieve easy Simple purification and preparation method, and the effect of improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
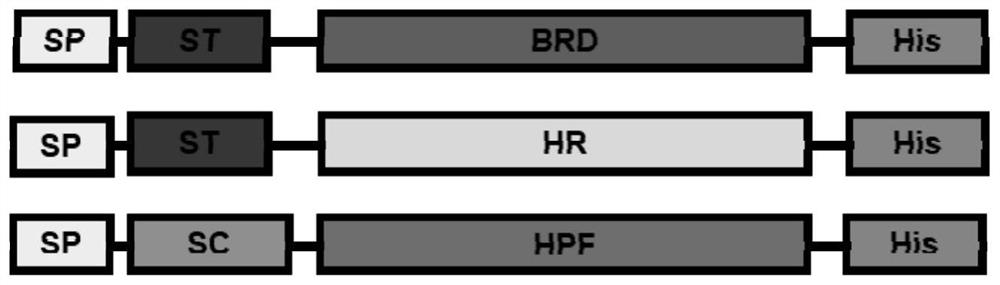
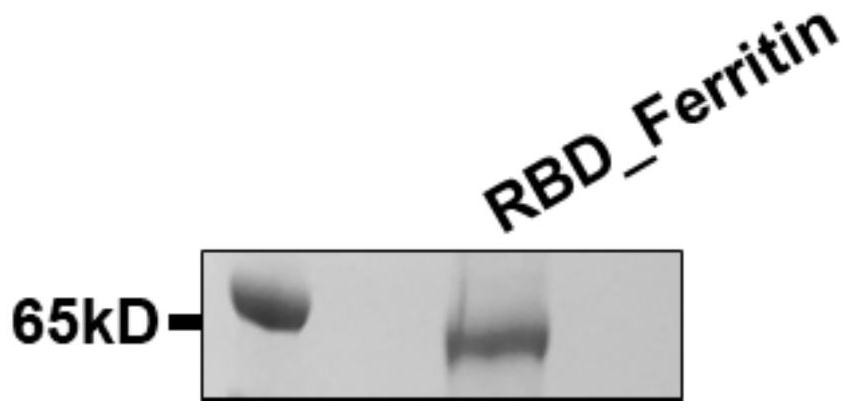
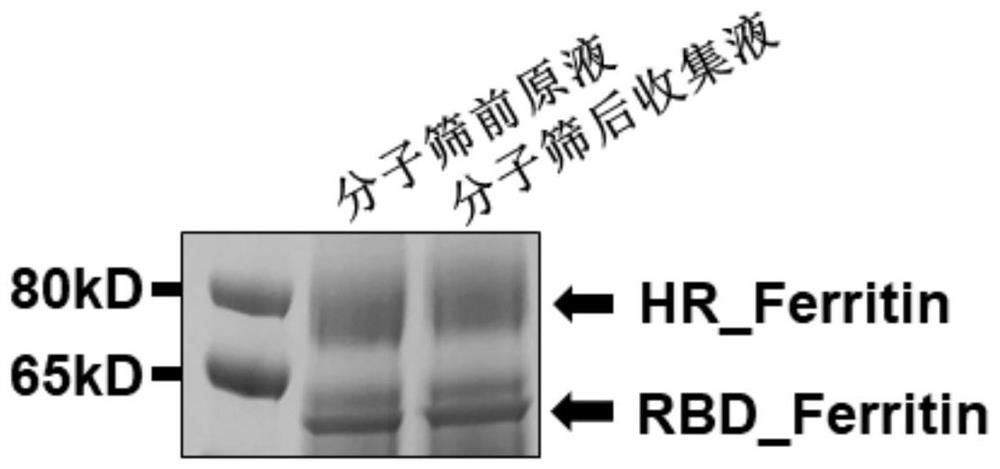
[0067] Example 1 Construction of novel coronavirus SARS-CoV-2 antigen (fusion protein RBD-HPF and RBD-HR-HPF)
[0068] Electron microscope counterstaining images of RBD-HPF subunit multimer protein and RBD-HR-HPF double subunit multimer protein self-assembled into nanoparticles figure 1 .
[0069] Specifically, the construction and preparation method of RBD-HPF subunit multimeric protein is as follows:
[0070] 1. Preparation of expressed His-tag-ST-RBD protein
[0071] Add a translation stop codon to the 3' end of the nucleotide sequence corresponding to SP-His-tag-ST-RBD and clone it into an expression vector (pcDNA3.1-Intron-WPRE) that adds Intron and WPRE to enhance expression to construct an expression vector .
[0072] DH5αcompetent cells were transformed with the recombinant plasmid, cultured overnight at 37°C, and positive clones were identified by screening and PCR. The endotoxin-free plasmid was extracted and used for the expression of nanoantigen protein after e...
Embodiment 2
[0082] Embodiment 2 mouse immunization experiment
[0083] The RBD-HPF protein and RBD-HR-HPF protein obtained in Example 1 were diluted to 100 μg / ml with physiological saline according to Table 1, and emulsified in groups with an equal volume of adjuvant SAS. Then BALB / c mice aged 6-8 weeks were immunized in groups. By intraperitoneal injection, each mouse was immunized with the vaccine three times on day 0, week 3 (day 21), and week 14 (day 108), with an inoculation volume of 200 μl (10 μg) each time. On the 10th, 31st, and 108th days, blood was collected from the mice's orbits. The mouse serum was obtained by centrifuging at 2800rpm at 4°C for 15 minutes after standing for a period of time to allow the serum to separate out, and was immediately used in the SARS-CoV-2 virus neutralization detection experiment.
[0084] Table 1
[0085] Antigen / Control Antigen content Adjuvant Number of animals (only) RBD-HPF protein 10μg SAS 4 RBD-HR-HPF pro...
Embodiment 3
[0086] Embodiment 3 Pseudovirus neutralization test
[0087] 1. Experimental method
[0088] Day 0:
[0089] Vero E6 cells were divided into 2 × 10 per well 4 cells at a density of 96 wells.
[0090] Day 1: (Wait for cell growth density to reach 100%.)
[0091] (1) Dilute serum: Serum is inactivated at 56°C for 30 minutes (this inactivation step is generally performed immediately after obtaining the serum), and the serum is diluted 5 times or 10 times according to the experimental requirements. Here, two dilution gradients are selected, namely 100 times and 1000 times. times. For example, to obtain a serum system with a final concentration of 10 times, 100 times and 100 times dilution, dilute as shown in Table 2 below:
[0092] Table 2
[0093]
[0094] (2) Dilute virus: 6000-8000 FFU per milliliter of DMEM culture medium, here, according to our laboratory experience, add 0.25ul of virus stock solution per well. Prepare a sufficient amount of diluted virus solution acc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com