Quality detection method of liver protection tablets
A quality inspection method and technology of Hugan Tablets, which are applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of single-component content determination, single-fingerprint, and difficulty in controlling the quality of single-flavor medicinal materials by fingerprints.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
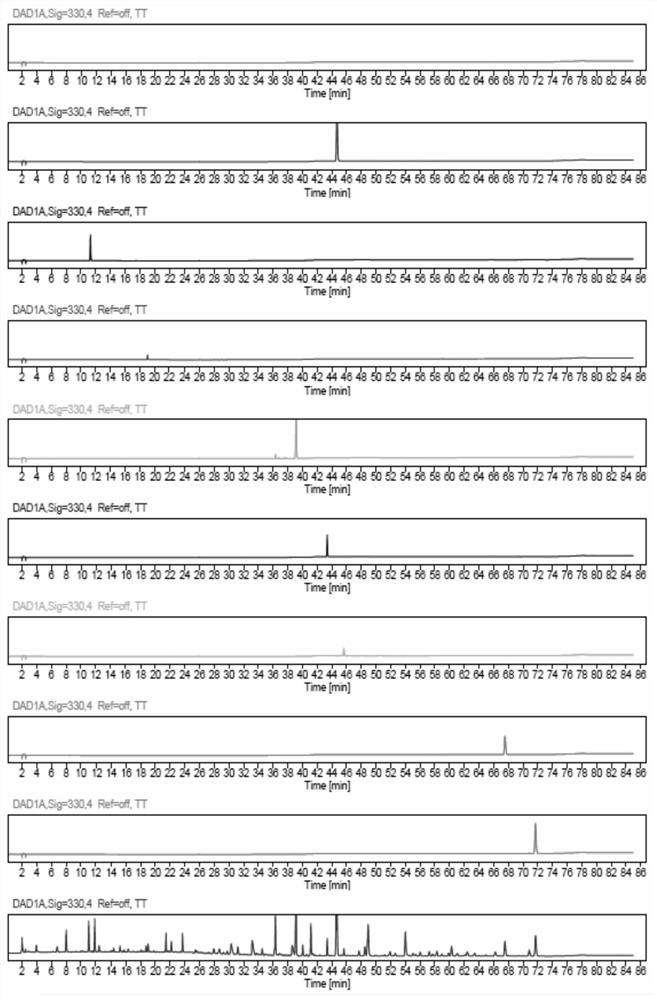
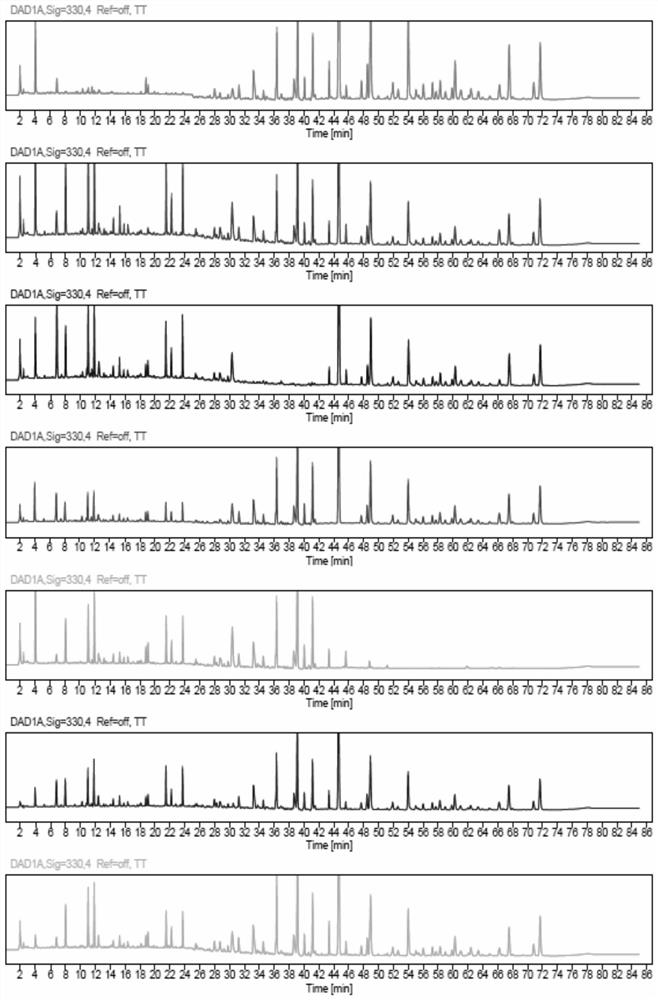
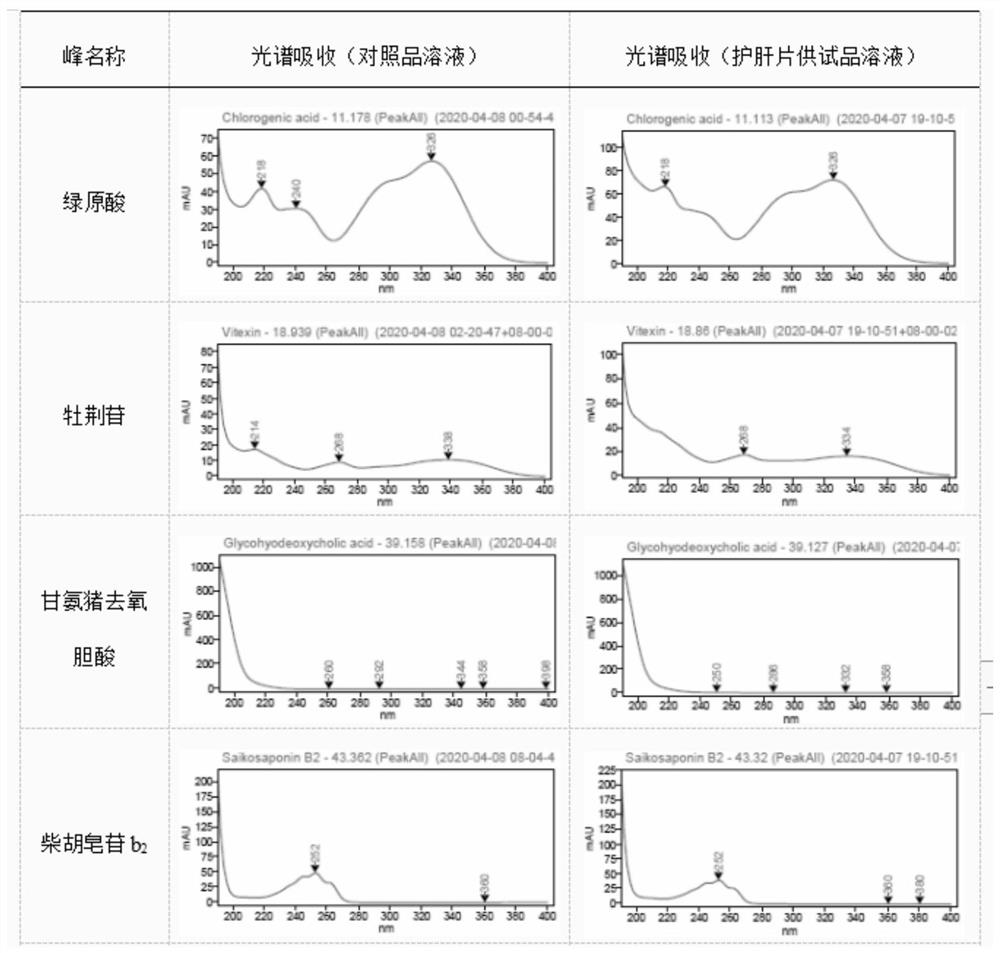
[0040] This embodiment provides a quality detection method for Hugan Tablets, that is, to detect the fingerprints and multi-component content of Hugan Tablets. The specific steps for the fingerprint determination of Hugan Tablets are as follows:
[0041] Instruments: Waters e2695 (PDA detector); Thermo U3000 (PDA detector); Agilent 1260 (VWD detector); Thermo U3000-QE high-resolution mass spectrometer; electronic balance (1 / 100,000), XSE105DU, Mettler -Toledo; electronic balance (1 / 10,000), MS204TS / 02, Mettler-Toledo; ultrasonic instrument, P120H, ElmaSchmidbauer GmbH, Germany; ultrapure water preparation instrument, Elix Advantage 15, Merck Millipore.
[0042]Material: liver protection tablet (batch number: 201705024, 201903092, 201903034, 201902044, 201902037, 201902021, 201902020, 201812170, 201812052, 201710026, 201707026), lack of five flavors Bile powder negative preparation, Heilongjiang Sunflower Pharmaceutical Co., Ltd.
[0043] Reference substances: Schizandrin A, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com