Crystal form a of 2-(2,5-dioxopyrrolidin-1 base) ethyl methyl fumarate and its preparation method and application
A technology of ethyl methyl fumarate and dioxopyrrolidine, which is applied to the crystal form A of 2-ethyl methyl fumarate and its preparation field, can solve the problem that it cannot be used as a pharmaceutical raw material and a product Physical state instability and other problems, to achieve good light stability, mild conditions, good efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Characterization of bulk drug crystal properties
[0088] 2-(2,5-Dioxopyrrolidin-1-yl)ethyl methyl fumarate was purchased from Shanghai Haoyuan Biomedical Technology Co., Ltd., and the chemical purity was greater than 98%.
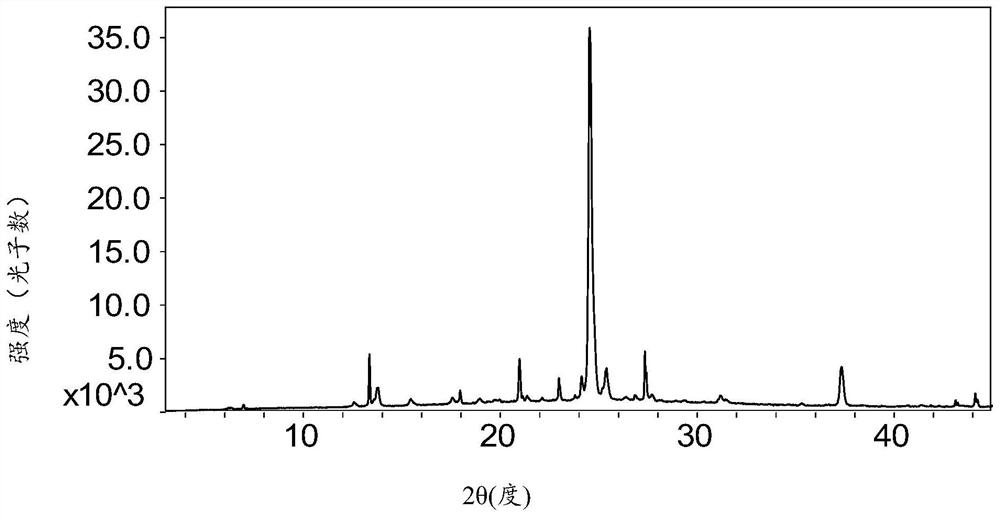
[0089] The XRPD pattern of the obtained API is as follows: figure 1 As shown, the characteristic peaks are shown in the following table, indicating that the compound prepared according to the method disclosed in the patent document CN105452213B is in a crystalline state, and is denoted as crystal form I.
[0090] Table 1 Characteristic peaks of crystal form I
[0091]
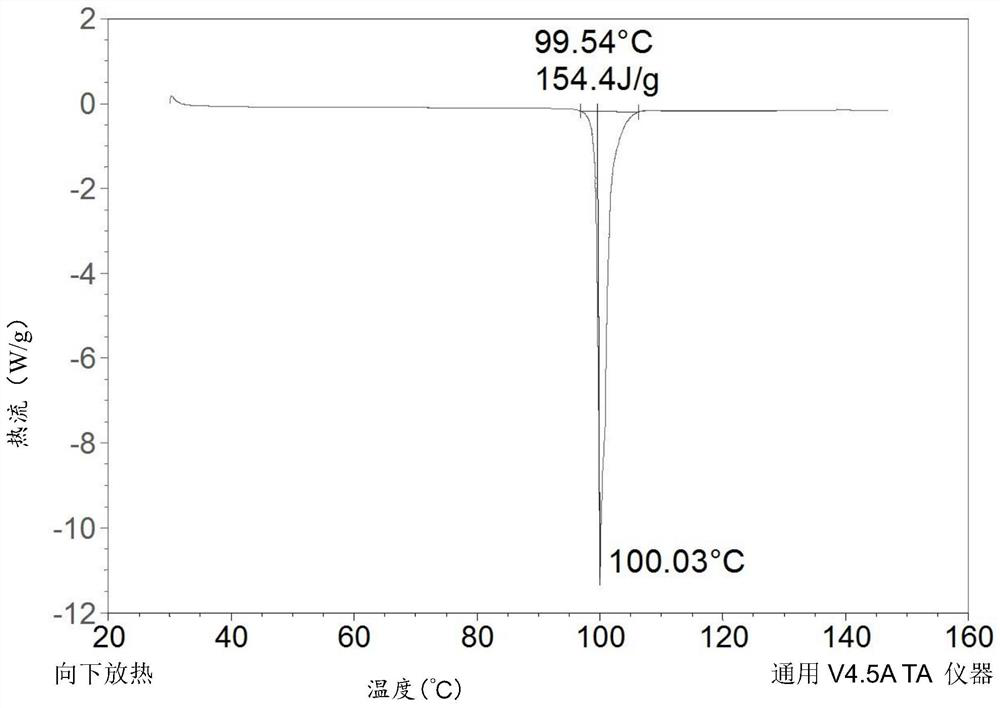
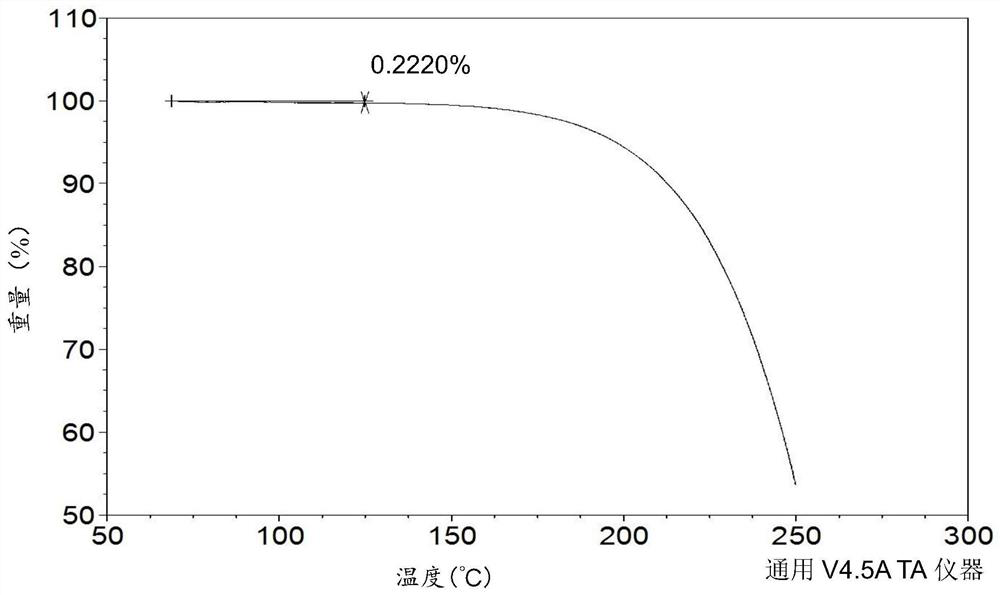
[0092] Further detection of the DSC and TGA curves of the drug substance such as figure 2 and 3 As shown, in the DSC curve, there is an endothermic peak at 94.0-107.0°C, and in the TGA curve, the weight loss of crystal form I before 125°C is 0.2220%.
Embodiment 2
[0093] Example 2 Preparation of Crystal Form A
[0094] Weigh 10.8 mg of raw materials into a sample bottle, add 0.4 ml of methanol to dissolve and clarify, slowly volatilize to obtain a solid, and vacuum dry at room temperature to obtain a white powder. The measured XRPD pattern is shown in Figure 4 The characteristic peaks are shown in the table below.
[0095] Table 2 Characteristic peaks of crystal form A
[0096]
[0097]
[0098] Further detection of the DSC and TGA curves of the white powder is as follows Figure 5 and 6 As shown, in the DSC curve, the crystal form A has an absorption peak at 96.0-107.0°C, and in the TGA curve, the weight loss of the crystal form A before 125°C is 0.002563%, indicating that the crystal form A is an ansolvate.
Embodiment 3
[0099] Example 3 Preparation of Crystal Form A
[0100] Weigh 8.3 mg of raw materials into a sample bottle, add 0.2 ml of ethyl acetate to dissolve and clarify at room temperature, slowly volatilize to obtain a solid, and vacuum dry at room temperature to obtain a white powder. The measured XRPD pattern is shown in Figure 7 shown, as in Example 2 Figure 4 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com