PH-responsive adriamycin carrier-free nano-drug as well as preparation method and application thereof
A nano-drug and doxorubicin technology, applied in the field of biomedical nano-materials, can solve the problems of low drug loading and high toxicity of organic solvents, and achieve the effects of clear components, easy quality control, and green and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of pH-responsive doxorubicin carrier-free nanomedicine
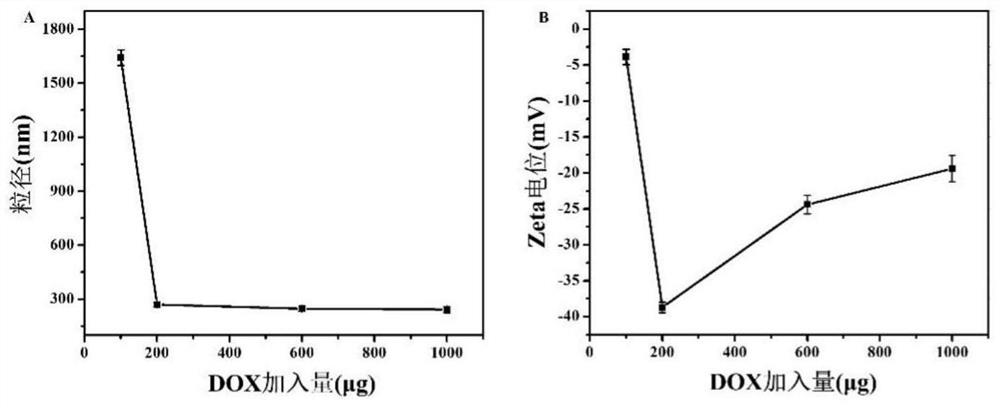
[0032] (1) Take a brown reaction bottle, first add 1.0mL of 1.0M sodium citrate solution, then add 200μg of DOX to it, and use an ultrasonic instrument to fully dissolve and mix the material evenly;
[0033] (2) The above mixed solution was placed on an infrared heating electromagnetic stirrer at room temperature and protected from light for 6 hours;
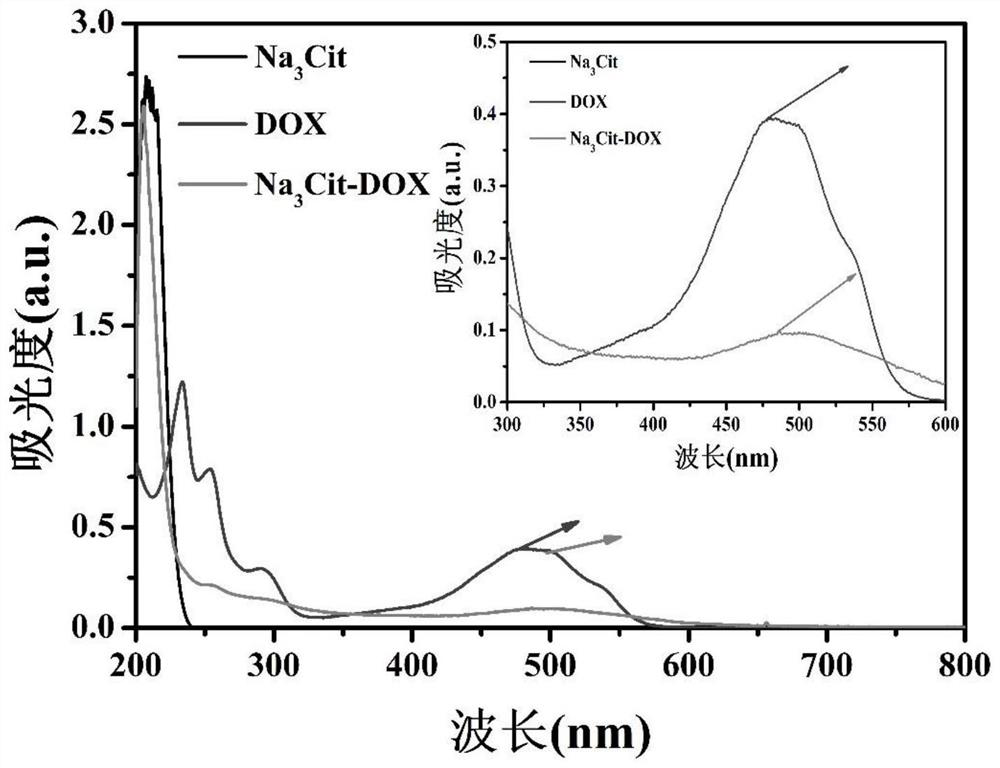
[0034] (3) Centrifuge at 12000rpm at high speed after the reaction is finished, and wash 4 times with sodium citrate solution, and freeze-dry to obtain the target product after washing. Collect all washing solutions in brown reagent bottles and store them in the dark until the drug loading of doxorubicin is determined. The absorbance of doxorubicin at 486nm was detected by ultraviolet-visible spectroscopic method, and the adsorption amount of DOX was calculated. The calculation shows that the loading rate of doxorubicin can be as high as 94.25±3.43%.
Embodiment 2
[0036] Preparation of pH-responsive doxorubicin carrier-free nanomedicine
[0037] (1) Take a brown reaction bottle, first add 1.0mL of 1.0M sodium citrate solution, then add 600μg DOX to it, and use an ultrasonic instrument to fully dissolve and mix the material evenly;
[0038] (2) The above mixed solution was placed on an infrared heating electromagnetic stirrer at room temperature and protected from light for 6 hours;
[0039] (3) Centrifuge at 12000rpm at high speed after the reaction is finished, and wash 4 times with sodium citrate solution, and freeze-dry to obtain the target product after washing. Collect all washing solutions in brown reagent bottles and store them in the dark until the drug loading of doxorubicin is determined. The absorbance of doxorubicin at 486nm was detected by ultraviolet-visible light spectrometry, and the adsorption amount of DOX was calculated. The calculation shows that the loading rate of doxorubicin can be as high as 94.85%±3.12%.
Embodiment 3
[0041] Preparation of pH-responsive doxorubicin carrier-free nanomedicine
[0042] (1) Take a brown reaction bottle, first add 1.0mL of 1.0M sodium citrate solution, then add 1000μg of DOX to it, and use an ultrasonic instrument to fully dissolve and mix the material evenly;
[0043] (2) The above mixed solution was placed on an infrared heating electromagnetic stirrer at room temperature and protected from light for 6 hours;
[0044] (3) Centrifuge at 12000rpm at high speed after the reaction is finished, and wash 4 times with sodium citrate solution, and freeze-dry to obtain the target product after washing. Collect all washing solutions in brown reagent bottles and store them in the dark until the drug loading of doxorubicin is determined. The absorbance of doxorubicin at 486nm was detected by ultraviolet-visible spectroscopic method, and the adsorption amount of DOX was calculated. The calculation shows that the loading rate of doxorubicin can be as high as 95.76±1.93%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com