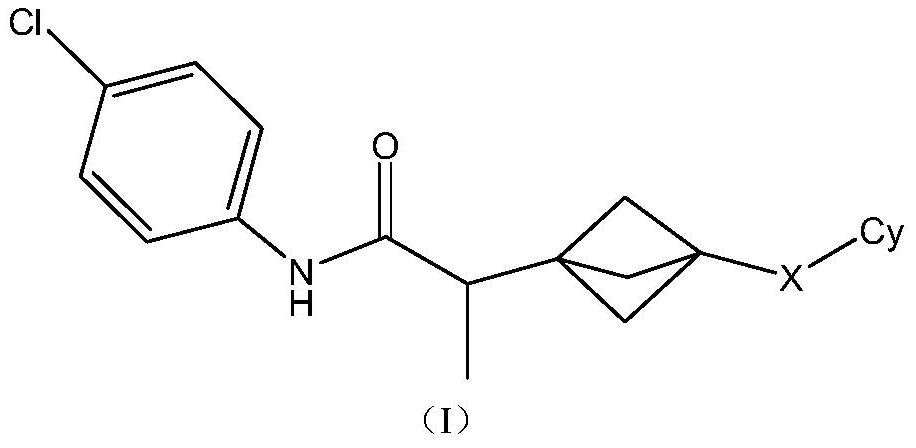
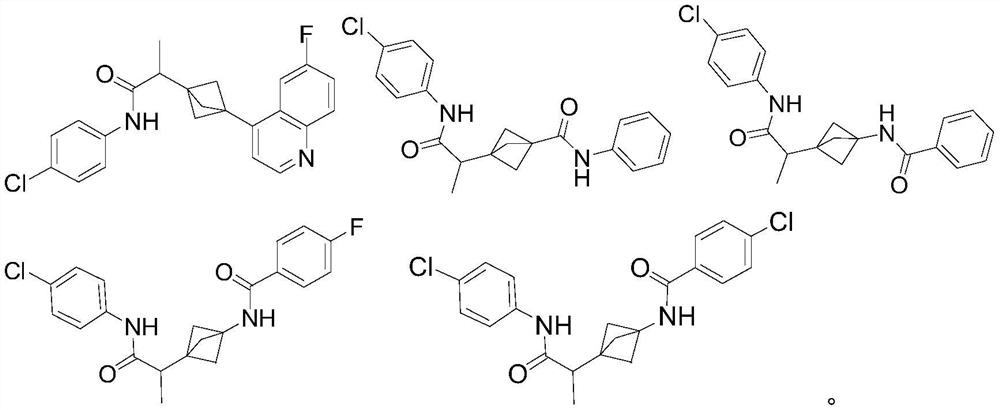
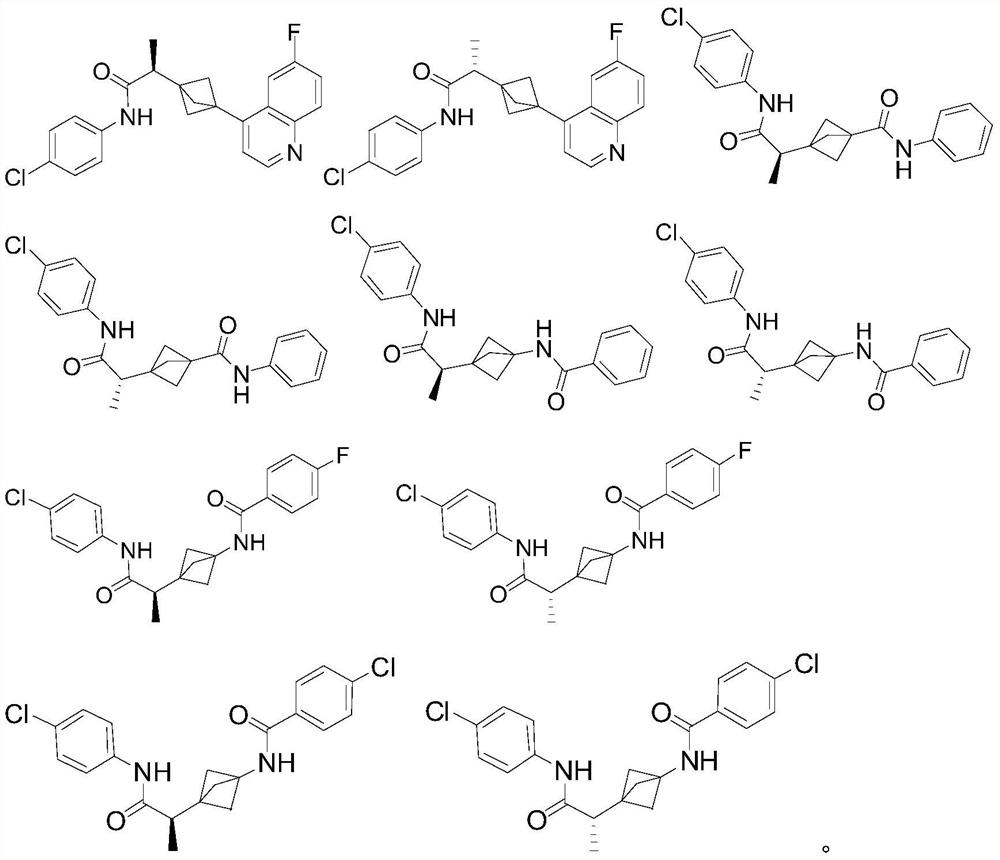
Indoleamine 2,3-dioxygenase inhibitor
A solvate and compound technology, applied in the field of infectious diseases, treatment of proliferative diseases, immune-related diseases and/or inflammatory diseases, can solve problems such as immune tolerance does not work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] N-(4-chlorophenyl)-2-[3-(6-fluoroquinolin-4-yl)bicyclo[1.1.1]pentane-1-yl]propanamide 1 (enantiomer, peak 1)
Embodiment 2
[0109] N-(4-chlorophenyl)-2-[3-(6-fluoroquinolin-4-yl)bicyclo[1.1.1]pentane-1-yl]propanamide 2 (enantiomer, peak 2)
[0110]
[0111] synthetic route
[0112] first step:
[0113]
[0114]3-(Methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid (CAS#83249-10-9, 4.5g, 26.6mmol, 1.0equiv), 6-fluoroquinoline (1.9g, 13.3 mmol, 0.5equiv), silver nitrate (903mg, 5.3mmol, 0.2equiv), copper acetate (241mg, 1.3mmol, 0.05equiv), potassium persulfate (7.2g, 27mmol, 1.0equiv) were sequentially added to the 250mL reaction flask, and then added Acetonitrile (70 mL) and water (30 mL) were stirred uniformly at room temperature, then heated to 60° C. and stirred for 2.5 h. After the reaction is complete, cool down to room temperature, spin to remove acetonitrile, add a small amount of water to dilute, extract three times with ethyl acetate, combine the organic phase, dry the organic phase with anhydrous sodium sulfate for 15min, filter to remove anhydrous sodium sulfate, and spin th...
Embodiment 3
[0146] (±) 3-[1-[(4-chlorophenyl)carbamoyl]ethyl]-N-phenylbicyclo[1.1.1]pentane-1-carboxamide 3 (racemate)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com