Pregabalin capsule and preparation method thereof
A technology of pregabalin and capsules, which is applied in the field of medicine, can solve the problems of poor patient compliance, poor fluidity of the total mixed powder, and low material yield, and achieve stable dissolution behavior, good fluidity, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The prescription and technology of embodiment 1 pregabalin capsule
[0022] The prescription is as follows:
[0023] components Proportion% Dosage mg per tablet Pregabalin 25.0 25.0 Silicified microcrystalline cellulose 75.0 75.0
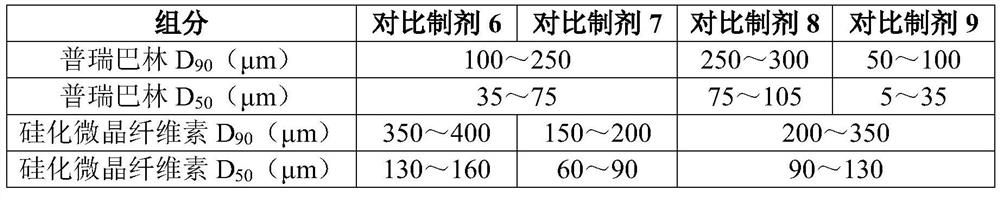
[0024] Choose pregabalin particle size distribution: D 90 100~250μm, D 50 35~75μm; choose the particle size distribution of silicified microcrystalline cellulose: D 90 200-350 μm; D 50 It is 90-130 μm.
[0025] The preparation method is as follows:
[0026] (1) Mix the pregabalin and the silicified microcrystalline cellulose of the prescribed amount evenly to obtain the total mixed granules for subsequent use;
[0027] (2) The weight loss on drying of the blended granules obtained in step (1) is ≤3.0%;
[0028] (3) Filling, polishing, and blistering the dry mixed powder in step (2) to obtain pregabalin capsules.
Embodiment 2-4
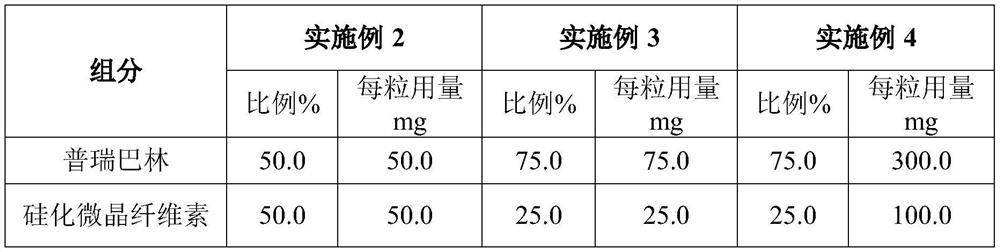
[0029] The prescription and technology of embodiment 2-4 pregabalin capsule
[0030] The prescription is as follows:
[0031]
[0032] The respective particle size distributions of pregabalin and silicified microcrystalline cellulose are the same as in Example 1;
[0033] The preparation method is the same as in Example 1, except that the dosages of pregabalin and silicified microcrystalline cellulose in step (1) are replaced by the prescription quantities in Examples 2-4.
Embodiment 5
[0034] Embodiment 5 comparative preparation and relevant test experiment
[0035] In order to illustrate the purpose of the formulation screening of the present invention, this example summarizes the comparative preparation formulations based on Examples 1-4 (the process is the same as that of Example 1), and the test experiments of the related properties of the formulations.
[0036] 1. Comparative preparations:
[0037] The content of the prescription of comparative preparation 1 is the same as that of Example 4, only the adjuvant silicified microcrystalline cellulose in Example 4 is replaced by lactose;
[0038] The content of the prescription of comparative preparation 2 is the same as that of Example 4, only the adjuvant silicified microcrystalline cellulose in Example 4 is replaced by microcrystalline cellulose;
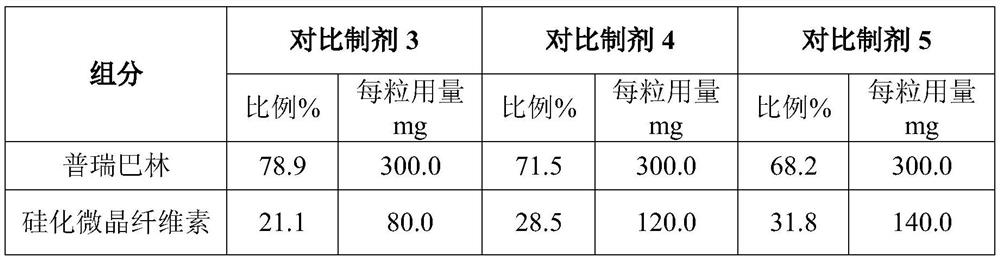
[0039] Contrast preparation 3-5 is all identical with the specification of embodiment 4 (300mg), improves the consumption ratio of raw material by reducing th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com