Application of amide compound in preparation of drug for treating sepsis
A technology of amide compounds and sepsis, applied in the field of application of amide compounds in the preparation of drugs for the treatment of sepsis, to achieve the effect of inhibiting cell pyroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
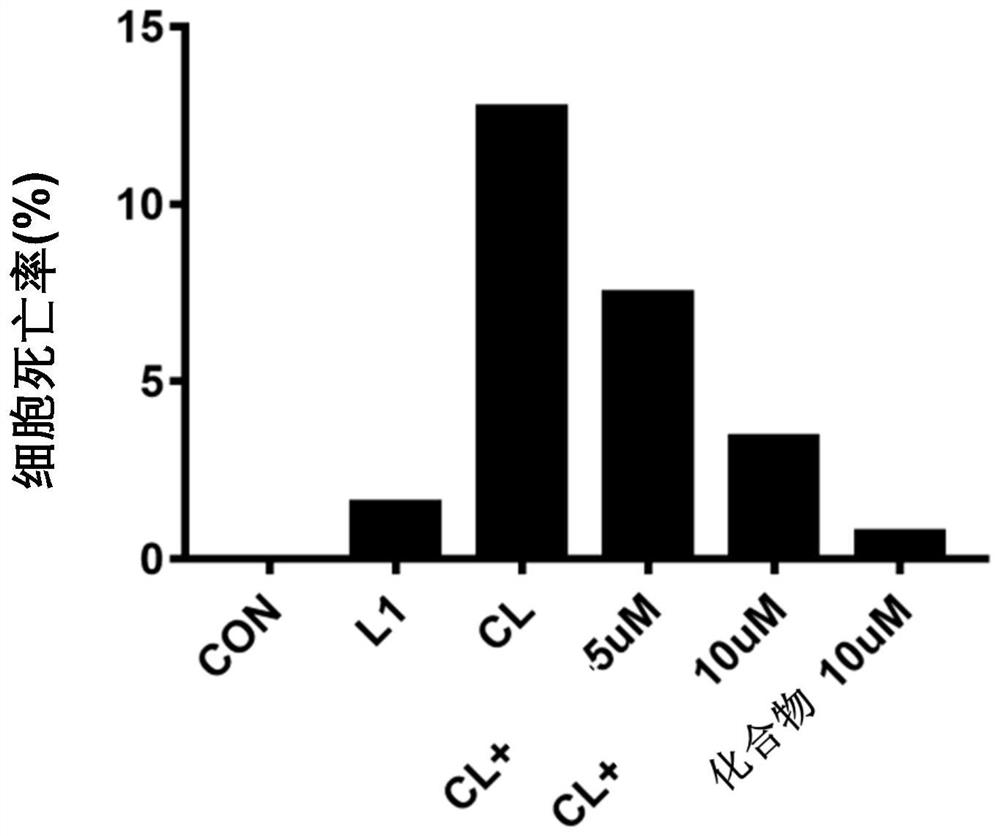
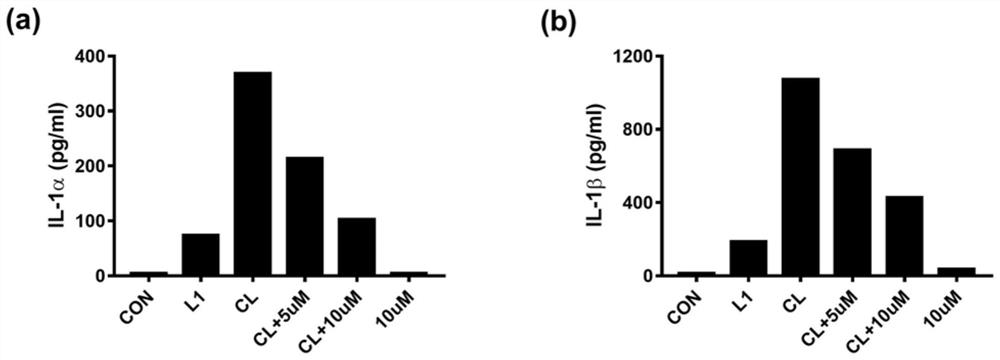
[0048] Example 1: Compounds inhibit primary macrophage pyroptosis induced by CTB transfection LPS activation Caspase11
[0049] 1. Experimental steps: Extract primary peritoneal macrophages from WT mice, resuspend cells in RPMI-1640 complete medium and adjust the concentration to 1×10 6 Cells / ml, 500 μl / well 24-well plate, wash the cells twice with DPBS after adhering to the wall overnight, and replace with serum-free 1640. The compounds were added to the cells at a final concentration of 5 μM and 10 μM for pre-incubation for 1 h, then the transfection reagent Cholera Toxin (CTB) 5 μg / ml+LPS 1 μg / ml (pre-incubated at room temperature for 20 min in advance) was added to stimulate the cells overnight, and the supernatant was collected for detection at 16-18 h Lactate dehydrogenase (LDH) content (to judge cell death rate) and ELISA detection (to detect pro-inflammatory cytokines IL-1α, IL-1β).
[0050] The specific experimental operation is as follows:
[0051] 1) Extract prima...
Embodiment 2
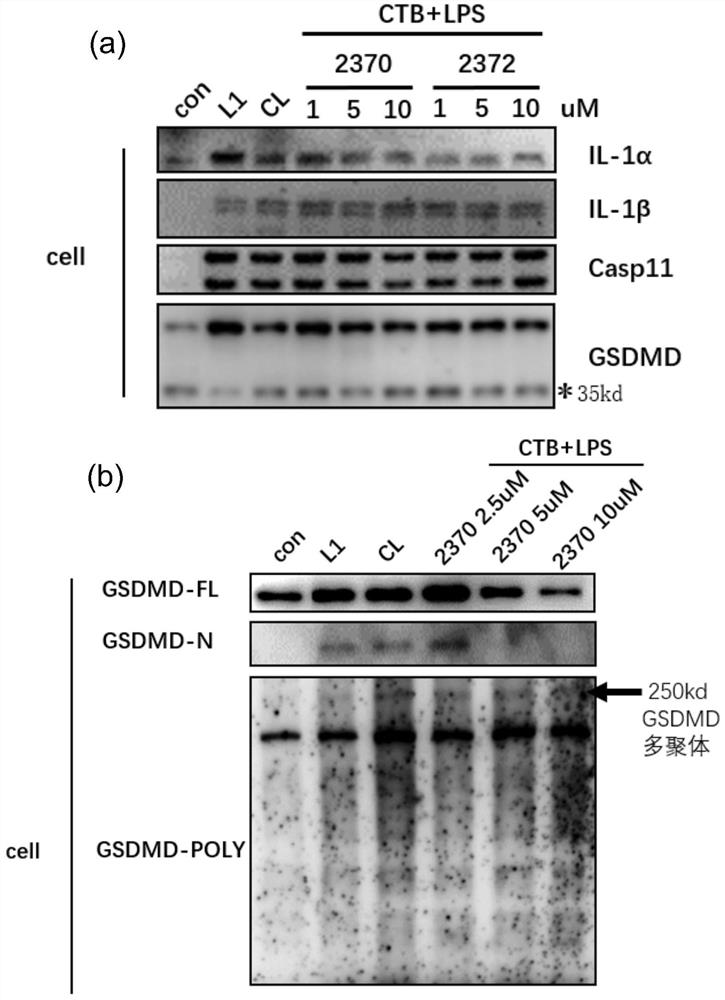
[0061] Example 2: Compounds inhibit the oligomerization of GSDMD and thereby inhibit the pyroptosis of primary macrophages
[0062]1. Experimental steps: Extract primary peritoneal macrophages from WT mice, resuspend cells in RPMI-1640 complete medium and adjust the concentration to 1×10^6 / ml, 2ml / well 6-well plate. After adhering to the wall overnight, the cells were washed twice with DPBS and replaced with serum-free 1640. Compounds were added to the cells at final concentrations of 5 μM and 10 uM for pre-incubation for 1 h, and then CTB 5 μg / ml+LPS 1 μg / ml (pre-incubated at room temperature for 20 min in advance) was added to stimulate the cells overnight. cocktail protease inhibitors and phosphatase inhibitors), lyse cell proteins on ice for 45min. Place the ice box on a shaker to ensure that the lysate fully contacts the cells. Then use a pre-cooled cell scraper to collect the cells into an EP tube, centrifuge at 13,000 g for 15 min at 4°C, and collect the supernatant a...
Embodiment 3
[0065] Example 3: Molecular docking and analog control experiments
[0066] 1. Experimental steps: through virtual screening, the compound is docked with GSDMD, and another analog is obtained through screening. The structural formula of the analog is: The binding energy of GSDMD after docking is reduced, identifying the docking active site. Among them, the 3D diagram of molecular docking of compounds and analogs with GSDMD protein is shown in Figure 4 shown.
[0067] 2. Structural analogue control experiment: the above-mentioned structural analogue was used to replace the compound in Example 1, and the pyroptosis inhibition experiment was carried out using the same method as in Example 1, and the dosing concentration was 10 μM.
[0068] 3. Experimental results: LDH detection experiment control results are as follows: Figure 5 as shown, Figure 5 Middle CON is the control group, and the addition amount of compounds and analogues in each group is 10 μM. After adding the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com