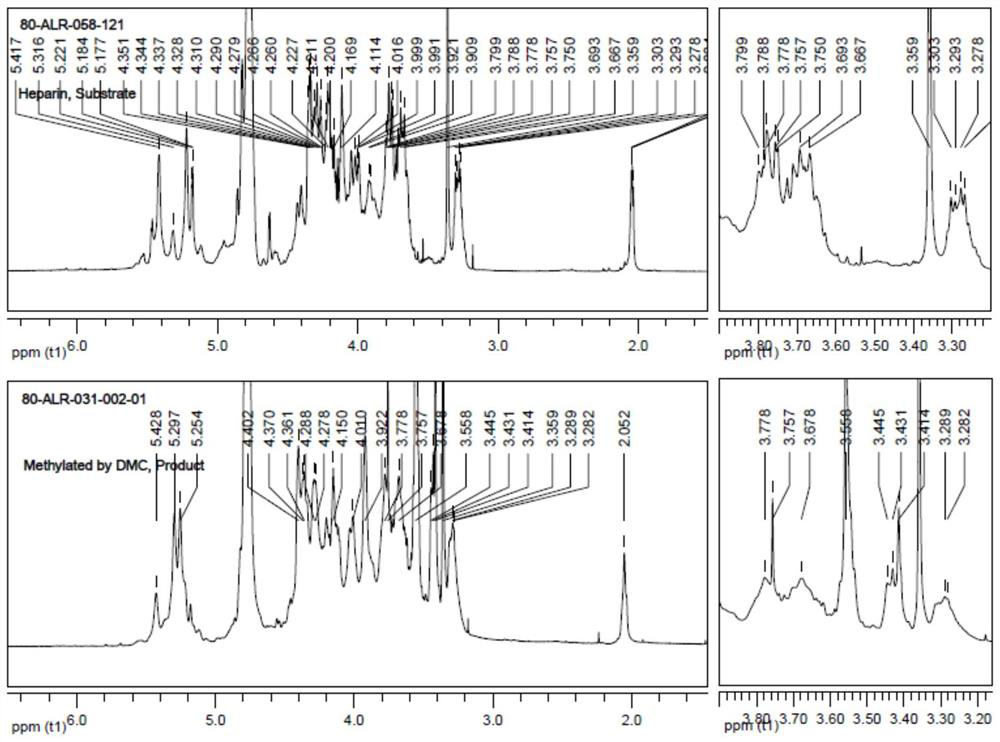
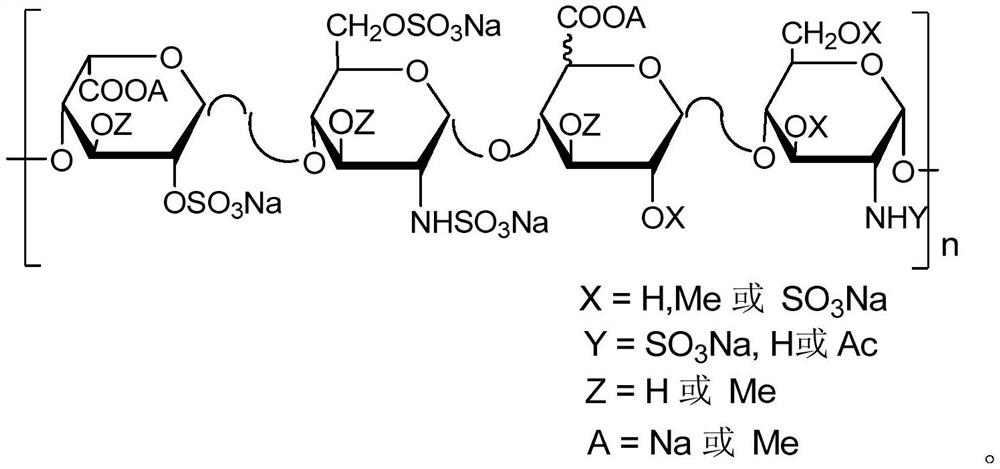
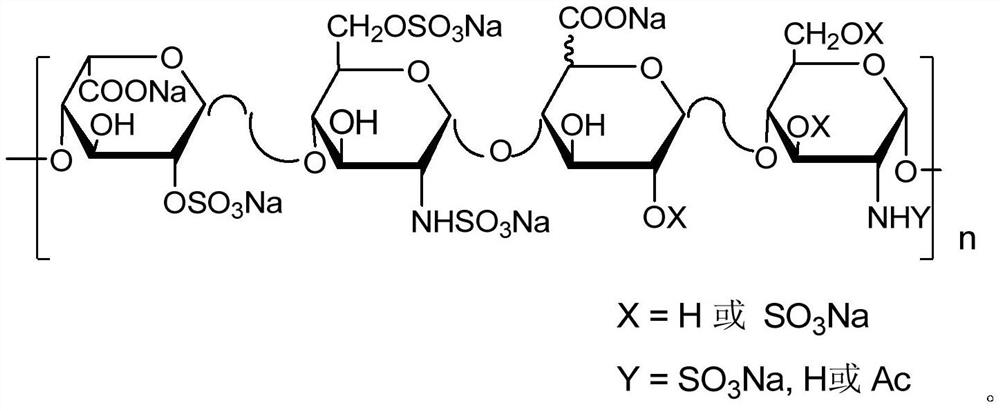
Methylated heparin compound as well as preparation and application thereof
A technology of methylated heparin and compounds, applied in the field of biomedicine, can solve the problems of poor medication comfort and achieve the effect of increasing the possibility and enriching the way of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Dissolve the heparin and prepare the heparin solution for the reaction. Specific steps are as follows:
[0027] Dissolution of heparin substrate: choose a 250mL three-neck round-bottom flask equipped with a thermometer and a nitrogen balloon, place the three-neck round-bottom flask in an ice-salt bath, add 1.5g heparin and 135mL purified water into the reaction flask, and start stirring to dissolve. After the heparin solution was completely dissolved, 15 mL of dichloromethane was added and the stirring was continued to prepare a heparin solution for reaction. The internal temperature of the heparin solution for reaction was -0.5°C.
[0028] 2. Heparin methylation:
[0029] Preparation of 30% sodium hydroxide aqueous solution: put 30mL of purified water in a 150mL plastic beaker, place the plastic beaker in an ice-salt bath, cool the purified water to ≤5°C, and slowly add 12.8g of sodium hydroxide solid Stir in purified water until the sodium hydroxide is completely...
Embodiment 2
[0039] 1. Dissolve the heparin and prepare the heparin solution for the reaction. Specific steps are as follows:
[0040] Dissolution of heparin substrate: prepare a 1000mL round-bottomed flask, configure one thermometer, one nitrogen balloon, and two 100mL constant-pressure dropping funnels, place the round-bottomed flask in an ice-salt bath, and mix 4.0g heparin with 360mL purified Add water into the reaction bottle, start stirring and dissolving, after the heparin solution is completely dissolved, add 40mL of chloroform, and continue stirring to prepare a heparin solution for reaction, the inner temperature of the heparin solution for reaction is -0.1°C.
[0041] 2. Heparin methylation:
[0042] Preparation of 30% sodium hydroxide aqueous solution by mass fraction: Put 100mL of purified water in a 500mL plastic beaker, place the plastic beaker in an ice-salt bath, cool down the purified water, and after the temperature stabilizes at 0.5-2.5°C, add 42.9g of hydrogen Slowly...
Embodiment 3
[0048] 1. Dissolve the heparin and prepare the heparin solution for the reaction. Specific steps are as follows:
[0049] Dissolution of heparin substrate: prepare a 10L round bottom flask, respectively configure one thermometer, one nitrogen balloon, one 800mL constant pressure dropping funnel, one 500mL constant pressure dropping funnel, place the round bottom flask in an ice-salt bath, Add 50.0g of heparin and 4500mL of purified water into the reaction bottle, and start stirring to dissolve. After the heparin solution is completely dissolved, add 500mL of chloroform and continue stirring to prepare a heparin solution for reaction. The internal temperature of the heparin solution for reaction is - 1.0°C.
[0050] 2. Heparin methylation:
[0051] Preparation of 30% sodium hydroxide solution by mass fraction: Put 1000mL purified water in a 5L plastic beaker, place the plastic beaker in an ice-salt bath, cool down the purified water to 2.0-3.5°C, and slowly dissolve 429g of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com