Preparation method of oral rehydration salts effervescent tablet
An effervescent tablet and rehydration technology, applied in the field of preparation of oral rehydration effervescent tablets, can solve the problems of poor stability, easy sticking of effervescent tablets, etc., and achieve the effects of solving poor stability, increasing compliance, and major innovation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: make 1000 oral rehydration salt effervescent tablets, its material consumption is as follows:
[0025] materials unit dose Sodium chloride 650g potassium chloride 375g citrate 473.6g anhydrous glucose 1900g sodium bicarbonate 621.3g lactose 330g essence 60g pigment 0.5g aspartame 10g Polyvinylpyrrolidone k30 320g PEG2000 210g
[0026] Preparation:
[0027] (1) Pulverize sodium chloride, potassium chloride, citrate, sodium bicarbonate, anhydrous glucose in the table above to particle size range 60-80 mesh respectively.
[0028] (2) Weigh the raw and auxiliary materials according to the prescription amount in the above table, and set aside.
[0029] (3) Add polyvinylpyrrolidone k30 and pigment to 80% ethanol aqueous solution by volume to prepare K30-pigment solution with 20% by mass.
[0030] (4) Put sodium chloride, potassium chloride, sodium bicarbonate, and anhydrous gluc...
Embodiment 2
[0037] Embodiment two: make 1000 oral rehydration salt effervescent tablets, its material consumption is as follows:
[0038] materials unit dose Sodium chloride 650g potassium chloride 375g citrate 473.6g anhydrous glucose 2050g sodium bicarbonate 621.3g lactose 240g essence 40g pigment 0.5g aspartame 10g Polyvinylpyrrolidone k30 340g PEG2000 200g
[0039] Preparation method is the same as embodiment one
[0040] The preparation process of the tablet was smooth, without stickiness, the disintegration time limit was 1.5min, and the solution was clear. Take 20 tablets and pulverize them, weigh a certain amount of pulverized products and dissolve them in purified water, and use atomic absorption spectrophotometer to detect the total sodium and potassium content. Measure 10 times. Content range: sodium 0.383~0.468, potassium 0.177~0.216
[0041] The result is as follows:
[0042] 1 2 ...
Embodiment 3
[0044] Embodiment three: make 1000 oral rehydration salt effervescent tablets, its material consumption is as follows:
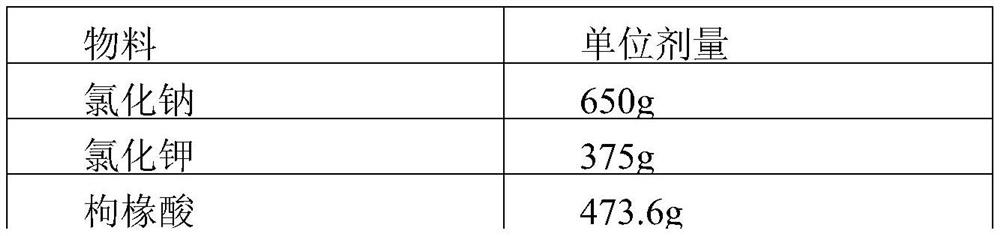
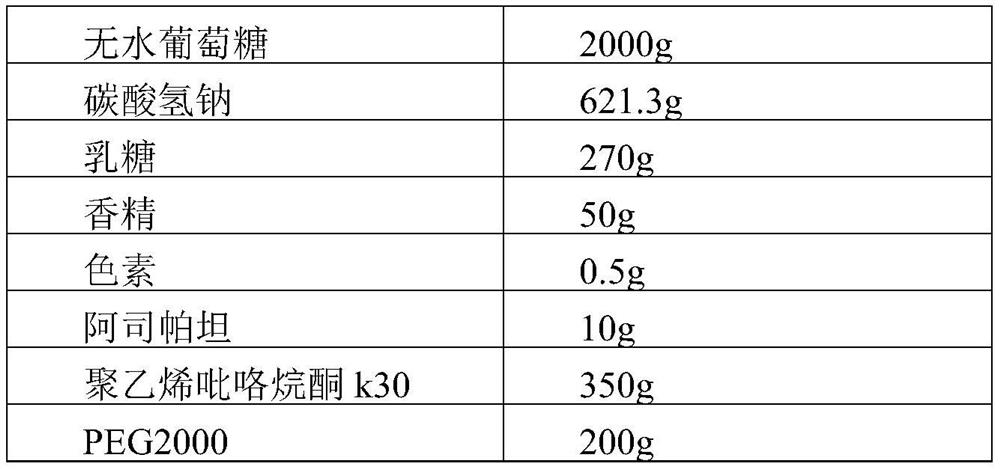
[0045]
[0046]
[0047] Preparation method is the same as embodiment one
[0048] The preparation process of the tablet was smooth, without stickiness, the disintegration time limit was 1.5min, and the solution was clear. Take 20 tablets and pulverize them, weigh a certain amount of pulverized products and dissolve them in purified water, and use atomic absorption spectrophotometer to detect the total sodium and potassium content. Measure 10 times.
[0049] The result is as follows:
[0050] 1 2 3 4 5 6 7 8 9 10 Total sodium (g) 0.4245 0.4233 0.4198 0.4133 0.4274 0.4112 0.4207 0.4102 0.4178 0.4159 Potassium (g) 0.1988 0.1898 0.1845 0.1975 0.1967 0.1940 0.1967 0.1856 0.1878 0.1903
[0051] The tablets are packaged and placed in a constant temperature and humidity box with a temperature of 40°C±2°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com