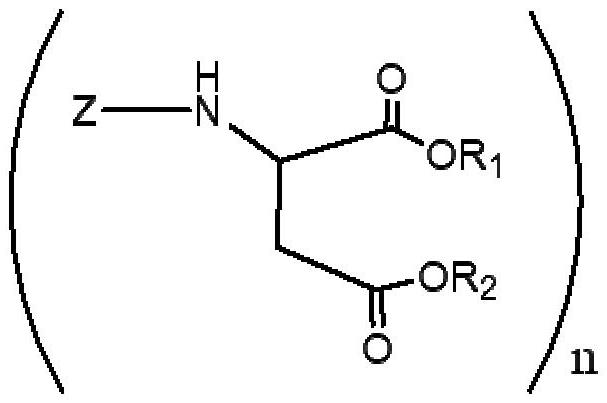
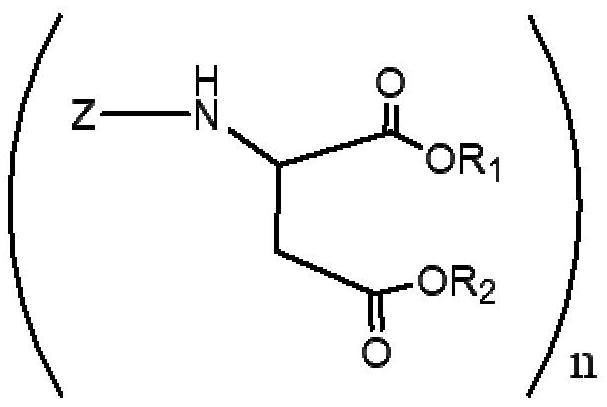
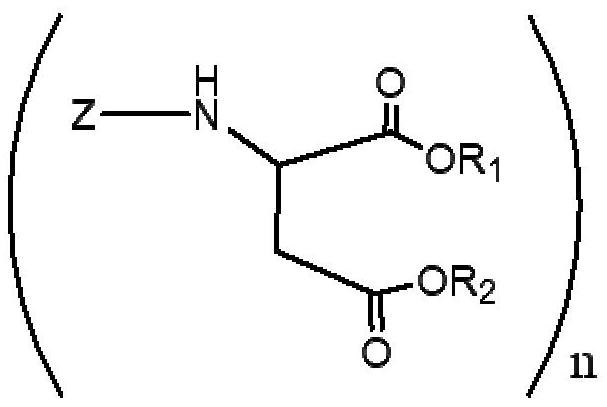
Polyurea compositions from polyaspartic esters and secondary heterocyclic amines derived aspartic esters
A technology of aspartic acid ester and composition, applied in the field of polyurea coating composition, can solve the problems of premature gelation of polyurea coating, application of mixture, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1. Preparation of a polyaspartate / aspartate mixture from diethyl maleate, 4,4'-diaminodicyclohexylmethane (PACM) and piperidine.
[0068] When equipped with N 2 A 2-liter glass reactor with gas inlet tube, thermocouple and addition funnel was charged with 4,4'-diaminodicyclohexylmethane (PACM) (470.72 g, 2.0 moles) and heated to 80°C. Diethyl maleate (688.8 g, 4 moles) was added slowly while maintaining the temperature at 80-85°C. The mixture was kept at this temperature for 6 hours. An oliquot sample analyzed by gas chromatography showed the presence of 5% by weight (0.32 moles) of diethyl fumarate. Piperidine (27.48 g, 0.32 mol) was added and the mixture was allowed to reach ambient temperature. After 3 days, no diethyl fumarate was detected by gas chromatography.
Embodiment 2
[0069] Example 2. Preparation of a polyaspartate / aspartate mixture from diethyl maleate, 4,4'-diaminodicyclohexylmethane (PACM) and pyrrolidine.
[0070] When equipped with N 2 A 2-liter glass reactor with gas inlet tube, thermocouple and addition funnel was charged with 4,4'-diaminodicyclohexylmethane (PACM) (470.72 g, 2.0 moles) and heated to 80°C. Diethyl maleate (688.8 g, 4 moles) was added slowly while maintaining the temperature at 80-85°C. The mixture was kept at this temperature for 6 hours. A sample analyzed by gas chromatography showed the presence of 5% by weight (0.32 moles) of diethyl fumarate. Pyrrolidine (22.90 g, 0.32 mol) was added and the mixture was allowed to reach ambient temperature. After 3 days, no diethyl fumarate was detected by gas chromatography.
Embodiment 3
[0071] Example 3. Preparation of a polyaspartate / aspartate mixture from diethyl maleate, 4,4'-diaminodicyclohexylmethane (PACM) and piperazine.
[0072] When equipped with N 2 A 2-liter glass reactor with gas inlet tube, thermocouple and addition funnel was charged with 4,4'-diaminodicyclohexylmethane (PACM) (470.72 g, 2.0 moles) and heated to 80°C. Diethyl maleate (688.8 g, 4 moles) was added slowly while maintaining the temperature at 80-85°C. The mixture was kept at this temperature for 6 hours. A sample analyzed by gas chromatography showed the presence of 5% by weight (0.32 moles) of diethyl fumarate. Piperazine (27.69 g, 0.32 mol) was added and the mixture was allowed to reach ambient temperature. After 7 days, no diethyl fumarate was detected by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com