Acetylene method vinyl chloride synthesis reaction process
An acetylene-based vinyl chloride synthesis reaction technology, which is applied in the field of warm reaction technology, can solve the problems of large number of reactors, complex reaction process, and increased reaction cost, so as to increase the effective heat exchange area and heat transfer capacity, and improve the reaction efficiency. Efficiency, the effect of saving equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
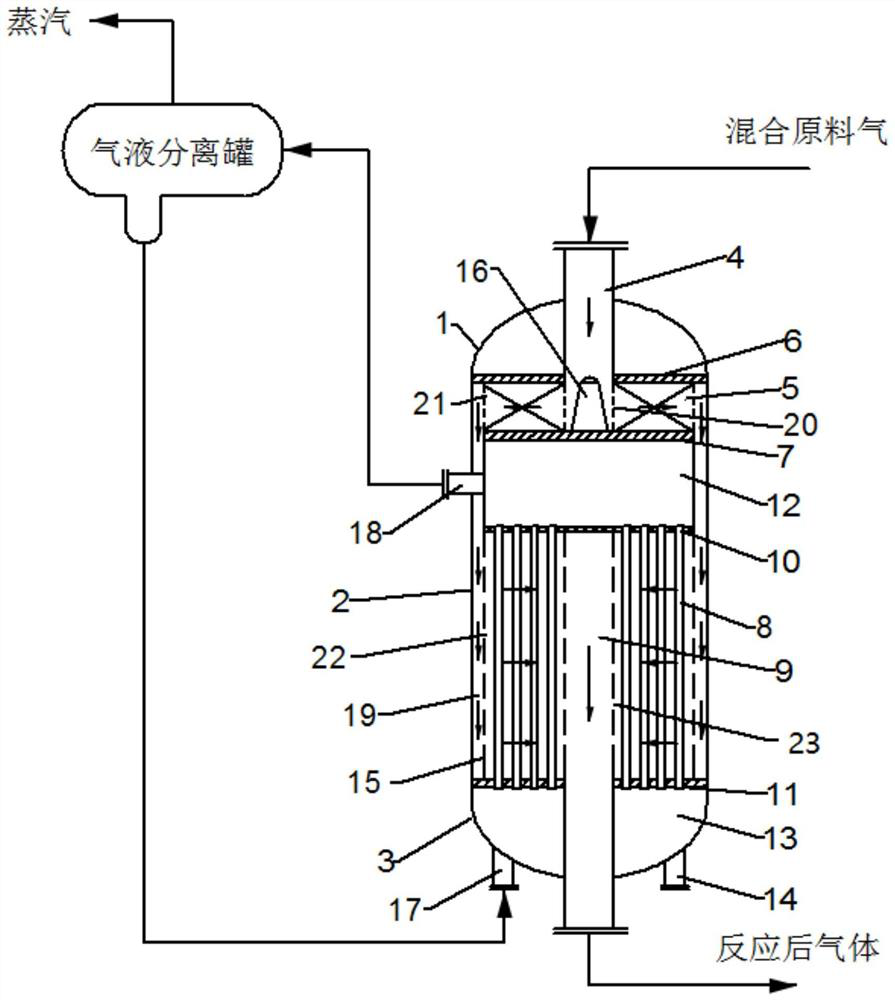
[0047] The raw material gas of the present invention enters the adiabatic reaction section of the catalyst from the central gas distribution pipe at the upper part of the reactor, enters the catalyst bed through the gas distributor, conducts the adiabatic catalytic reaction along the radial direction, and then enters the isothermal catalytic reaction section through the gas channel, and then passes through the shell. The openings of the catalyst bed enter the catalyst bed, carry out isothermal catalytic reaction along the radial direction, and finally enter the central gas collecting pipe to exit the reactor. The circulating medium enters the water chamber at the bottom of the reactor from the gas-liquid separator, enters the catalyst bed along the heat exchange tube, enters the gas chamber after absorbing heat and vaporizes, and returns to the gas-liquid separator.
[0048] Below in conjunction with the examples, the specific implementation of the present invention will be fur...
Embodiment 1
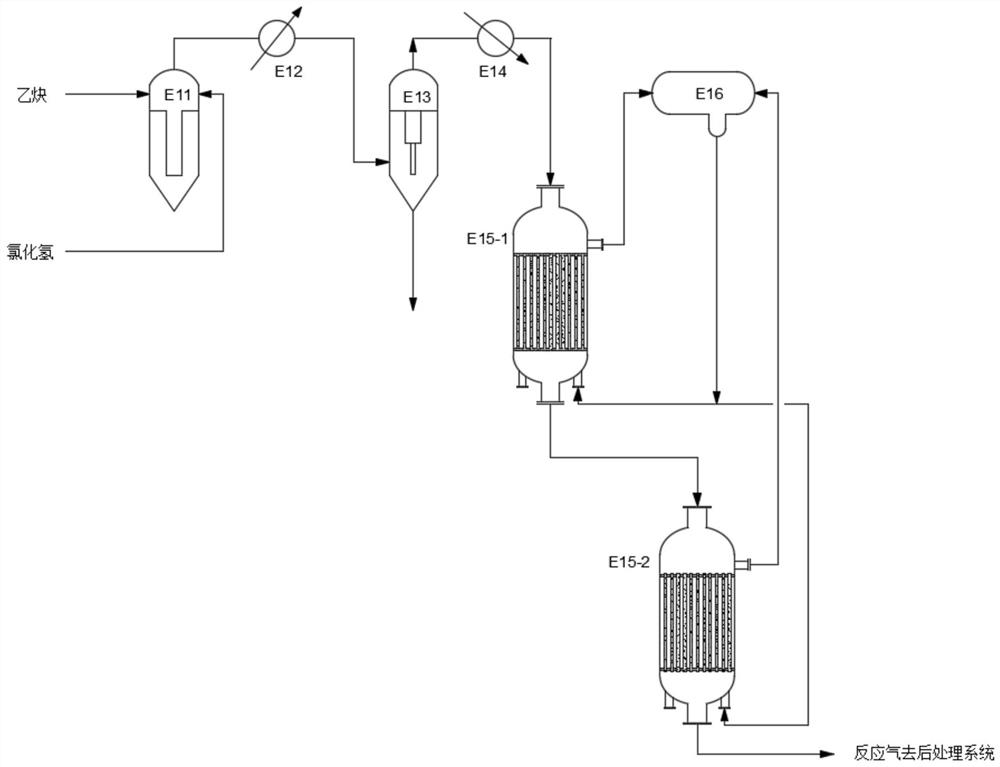
[0050] as attached figure 1 As shown, a vinyl chloride synthesis system using a radial adiabatic isothermal reactor connected in parallel, including a radial adiabatic isothermal reactor and a gas-liquid separator. Reactor E4 is an acetylene-process vinyl chloride synthesis reactor, which includes an upper head 1, a shell 2 and a lower head 3 from top to bottom, and is characterized in that the shell 2 includes an upper section, a Air chamber 12, lower section and water chamber 13.
[0051] The upper section includes a central air distribution pipe 4, a catalyst pressure plate 6, an adiabatic catalytic reaction section 5 and a support plate 7, the central air distribution pipe 4 is arranged in the middle of the upper section, the upper end is arranged outside the upper head 1, and the lower end is connected to the support plate 7, Between the catalyst pressure plate 6 and the support plate 7 is an adiabatic catalytic reaction section 5 . The lower section includes a heat exc...
Embodiment 2
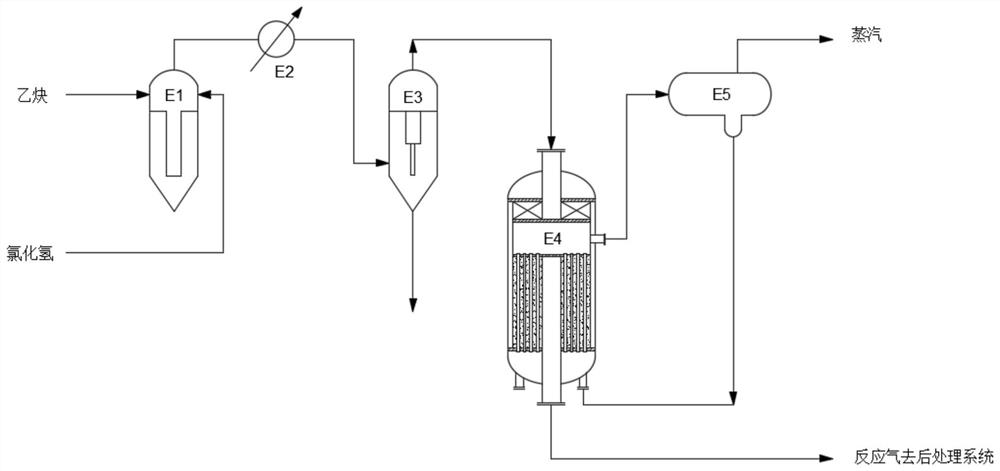
[0055] After the raw material gas hydrogen chloride and acetylene are mixed in the mixer E1 with a molar ratio of 1.1:1, the mixed raw gas is cooled to -14°C through the mixed gas cooler E2, and then the mixed gas is removed through the demister E3. Hydrochloric acid mist, then enters the reactor from the top of the radial reactor E4, and enters the adiabatic catalytic reaction section through the central gas distribution pipe. The gas channel and the gas distributor enter the isothermal catalytic reaction section, and then enter the catalyst bed through the openings on the shell, and carry out the isothermal catalytic reaction along the radial direction. The space velocity of the raw material gas acetylene entering the central gas distribution pipe is controlled at 50h -1 , The feed gas pressure is 40kPag. The mixed feed gas undergoes catalytic reaction along the radial direction, and finally enters the central gas collecting pipe and exits the reactor from the bottom of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com